Exam 15: Acids and Bases
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
Calculate the pH of a 0.20 M solution of the weak base pyridine (C5H5N; Kb = 1.7 x 10-9)
(Short Answer)
4.7/5  (36)
(36)
Arrange the acids HOBr, HBrO3, and HBrO2 in order of increasing acid strength.
(Multiple Choice)
4.8/5  (37)
(37)
A 2.1 L sample of a 0.23 M NaOH solution is mixed with 1.9 L of a 0.021 M KOH solution.What is the pH of the mixture?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following is both a Lewis Acid and Brønsted Acid?
(Multiple Choice)
4.8/5  (38)
(38)
The oxides SO2 and N2O5 will form the following acids in water, respectively.
(Multiple Choice)
4.9/5  (28)
(28)
Which one of these salts will form a basic solution upon dissolving in water?
(Multiple Choice)
4.9/5  (44)
(44)
The salt hydrolysis reaction for CH3COONa that represents the acidic or basic nature of the solution is:
(Multiple Choice)
4.8/5  (39)
(39)
Predict the direction in which the equilibrium will lie for the reaction H3PO4(aq)+ HSO4-(aq) 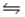 H2PO4-(aq) + H2SO4(aq).
Ka1(H3PO4)= 7.5 × 10-3; Ka(H2SO4)= very large
H2PO4-(aq) + H2SO4(aq).
Ka1(H3PO4)= 7.5 × 10-3; Ka(H2SO4)= very large
(Multiple Choice)
4.9/5  (31)
(31)
Which one of the following statements is true for a 0.1 M solution of a weak acid HA?
(Multiple Choice)
4.7/5  (32)
(32)
Write the chemical formula for the acid formed when Cl2O5 is dissolved in water.
(Short Answer)
4.9/5  (29)
(29)
Which of the following does not fit the definition of a Brønsted Acid?
(Multiple Choice)
5.0/5  (42)
(42)
In the reaction HNO3 + NH3 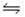 NH4+ + NO3-, NH4+ and NH3 are a conjugate acid-base pair.
NH4+ + NO3-, NH4+ and NH3 are a conjugate acid-base pair.
(True/False)
5.0/5  (43)
(43)
Calculate the H+ ion concentration in a 8.8 × 10-4 M Ca(OH)2 solution.
(Multiple Choice)
4.7/5  (31)
(31)
Arrange the acids HOCl, HClO3, and HClO2 in order of increasing acid strength.
(Multiple Choice)
4.9/5  (38)
(38)
In the reaction: 2H2O(l) 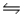 H3O+(aq)+ OH- (aq)the conjugate acid-base pairs are
H3O+(aq)+ OH- (aq)the conjugate acid-base pairs are
(Multiple Choice)
4.7/5  (37)
(37)
Showing 101 - 120 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)