Exam 15: Acids and Bases
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
What is the pH of a 0.11 M solution of C6H5OH (Ka = 1.3 x 10-10)
(Multiple Choice)
4.9/5  (42)
(42)
A 5.5 L sample of a 0.25 M HNO3 solution is mixed with 1.2 L of a 0.34 M HCl solution.What is the pH of the mixture?
(Multiple Choice)
4.7/5  (33)
(33)
Will a 0.1 M solution of NH4NO2(aq)be acidic, basic, or neutral?
(Short Answer)
4.8/5  (38)
(38)
What is the pH of a solution prepared by mixing 100.mL of 0.0500 M HCl with 300.mL of 0.500 M HF? [Ka(HF)= 7.1 × 10-4]
(Multiple Choice)
4.9/5  (33)
(33)
The OH- concentration in a 7.5 × 10-3 M Ca(OH)2 solution is
(Multiple Choice)
4.7/5  (29)
(29)
Calculate the H+ ion concentration in lemon juice having a pH of 2.40.
(Multiple Choice)
4.8/5  (35)
(35)
Which one of these net ionic equations represents the reaction of a strong acid with a weak base?
(Multiple Choice)
4.9/5  (27)
(27)
Calculate the pH of a 0.18 M solution of KNO2 (Ka (HNO2)= 4.5 x 10-4)
(Multiple Choice)
4.8/5  (37)
(37)
The pH of tomato juice is about 4.5.Calculate the concentration of hydrogen ions in this juice.
(Multiple Choice)
4.8/5  (36)
(36)
Predict the direction in which the equilibrium will lie for the reaction H3PO4 + NO3-
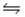 H2PO4- + HNO3 Ka(H3PO4)= 7.5 × 10-3
H2PO4- + HNO3 Ka(H3PO4)= 7.5 × 10-3
(Multiple Choice)
4.7/5  (28)
(28)
Which one of the following equations represents the ionization of a weak base in water?
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the pH of a 0.021 M NaCN solution.[Ka(HCN)= 4.9 × 10-10]
(Multiple Choice)
4.9/5  (38)
(38)
Which one of these salts will form a basic solution on dissolving in water?
(Multiple Choice)
4.7/5  (39)
(39)
Given the following Ka values, which anion is the strongest base? 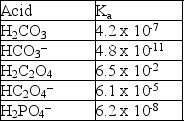
(Multiple Choice)
4.7/5  (39)
(39)
Which one of these equations represents the reaction of a weak acid with a weak base?
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the pH of a 0.011 M solution of NH4NO3 (Kb(NH3)= 1.8 x 10-5)
(Short Answer)
4.8/5  (40)
(40)
Showing 81 - 100 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)