Exam 15: Acids and Bases
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
A 5.2 L sample of a 1.1 M KOH solution is mixed with 2.3 L of a 0.20 M Sc(OH)3 solution.What is the pH of the mixture?
(Multiple Choice)
4.8/5  (37)
(37)
A 0.10 M NH3 solution is 1.3% ionized.Calculate the H+ ion concentration. NH3 + H2O 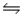 NH4+ + OH-
NH4+ + OH-
(Multiple Choice)
4.7/5  (26)
(26)
Calculate the H+ ion concentration in a solution with a pH of 3.85.
(Short Answer)
4.8/5  (42)
(42)
Consider the weak bases below and their Kb values: 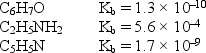 Arrange the conjugate acids of these weak bases in order of increasing acid strength.
Arrange the conjugate acids of these weak bases in order of increasing acid strength.
(Multiple Choice)
4.8/5  (35)
(35)
Consider the weak acid CH3COOH (acetic acid).If a 0.048 M CH3COOH solution is 5.2% ionized, determine the [H3O+] concentration at equilibrium.
(Multiple Choice)
4.8/5  (36)
(36)
Identify the conjugate acid-base pairs in the reaction HSO4- + HF 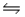 H2SO4 + F-
One conjugate acid-base pair is _______________; the other acid-base pair is ______________.
H2SO4 + F-
One conjugate acid-base pair is _______________; the other acid-base pair is ______________.
(Essay)
4.9/5  (34)
(34)
The pOH of a solution is 9.60.Calculate the hydrogen ion concentration in this solution.
(Multiple Choice)
4.8/5  (31)
(31)
What is the OH- ion concentration in a 5.2 × 10-4 M HNO3 solution?
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the pH of a 0.14 M HNO2 solution that is 5.7% ionized.
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following does not fit the definition of a Brønsted Base?
(Multiple Choice)
4.9/5  (40)
(40)
Rain collected on a remote island in the Pacific assumed to be unaffected by human pollution.The pH of the rainwater on this island was not 7.Do you expect the pH to be greater than 7 or less than 7? Explain your reasoning.
(Essay)
4.9/5  (29)
(29)
The pH of a 0.095 M solution of an unknown monoprotic acid is 5.42.Calculate the Ka of the acid.
(Multiple Choice)
4.8/5  (40)
(40)
Due to a highway accident, 150 L of concentrated hydrochloric acid (12.0 M)is released into a lake containing 5.0 × 105 m3 of water.If the pH of this lake was 7.0 prior to the accident, what is the pH of the lake following the accident?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following yields an acidic solution when dissolved in water? I.NO2
II.NH4Cl
III.NaCl
IV.HNO2
(Multiple Choice)
4.9/5  (39)
(39)
What is the pH of a 0.20 M solution of NH4Cl? [Kb(NH3)= 1.8 × 10-5]
(Multiple Choice)
4.9/5  (37)
(37)
The salt hydrolysis reaction for NH4NO3 that represents the acidic or basic nature of the solution is:
(Multiple Choice)
4.9/5  (37)
(37)
Showing 41 - 60 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)