Exam 15: Acids and Bases
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
Will a 0.1 M solution of NaH2PO4(aq)be acidic, basic, or neutral?
(Short Answer)
4.9/5  (39)
(39)
The pH of a certain solution is 3.0. How many H+(aq)ions are there in 1.0 L of the solution?
(Multiple Choice)
4.8/5  (37)
(37)
In the reaction Ag+(aq)+ Cl-(aq) AgCl(s), Ag+ acts as a Lewis acid.
(True/False)
4.8/5  (36)
(36)
When 2.0 * 10-2 mole of nicotinic acid (a monoprotic acid)is dissolved in 350.mL of water, the pH is 3.05.What is the Ka of nicotinic acid?
(Short Answer)
4.8/5  (34)
(34)
Calculate the pH of a 0.10 M HCN solution that is 0.0070% ionized.
(Multiple Choice)
4.8/5  (39)
(39)
What is the pH of an aqueous solution that contains 2.7 *1020 H3O+ ions per liter of solution?
(Multiple Choice)
4.8/5  (37)
(37)
Determine the pH of a KOH solution made by mixing 0.251 g KOH with enough water to make 1.00 * 102 mL of solution.
(Multiple Choice)
4.8/5  (38)
(38)
If the pH of an acid rain storm is approximately 3.0, how many times greater is the [H+] in the rain than in a cup of coffee having a pH of 5.0?
(Multiple Choice)
4.8/5  (29)
(29)
In 0.10 M KCN, the chemical species with the highest concentration (except H2O)is
(Multiple Choice)
4.9/5  (38)
(38)
Suppose that ammonia, applied to a field as a fertilizer, is washed into a farm pond containing 3.0 * 106 L of water. If the pH of this pond is found to be 9.81, what volume of liquid ammonia found its way into the pond? [Given: Kb(NH3)= 1.8 * 10-5; the density of liquid ammonia is 0.771 g/cm3]
(Multiple Choice)
5.0/5  (42)
(42)
For H3PO4, Ka1 = 7.3 * 10-3, Ka2 = 6.2 *10-6, and Ka3 = 4.8 *10-13. An aqueous solution of Na3PO4 therefore would be
(Multiple Choice)
4.9/5  (40)
(40)
Which one of these net ionic equations represents the reaction of a strong acid with a weak base?
(Multiple Choice)
4.9/5  (40)
(40)
Rain collected on a remote island in the Pacific assumed to be unaffected by human pollution.The pH of the rainwater on this island was not 7.Do you expect the pH to be greater than 7 or less than 7? Explain your reasoning.
(Essay)
4.9/5  (32)
(32)
Which one of these equations represents the reaction of a weak acid with a strong base?
(Multiple Choice)
4.8/5  (29)
(29)
Predict the direction in which the equilibrium will lie for the reaction H2SO3(aq)+ HCO3- (aq) 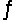 HSO3-(aq)+ H2CO3(aq).
Ka1(H2SO3)= 1 * 10-2; Ka1(H2CO3)= 4.2 * 10-7
HSO3-(aq)+ H2CO3(aq).
Ka1(H2SO3)= 1 * 10-2; Ka1(H2CO3)= 4.2 * 10-7
(Multiple Choice)
4.9/5  (29)
(29)
Arrange the acids HBr, H2Se, and H3As in order of increasing acid strength.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 81 - 100 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)