Exam 15: Acids and Bases
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
What is the pH of a 0.20 M solution of NH4Cl? [Kb(NH3)= 1.8 * 10-5]
(Multiple Choice)
4.9/5  (32)
(32)
What mass of potassium hypochlorite must be added to 450.mL of water to give a solution with pH = 10.20? [Ka(HClO)= 4.0 * 10-8]
(Multiple Choice)
5.0/5  (45)
(45)
The pH of coffee is approximately 5.0. How many times greater is the [H+] in coffee than in neutral water?
(Multiple Choice)
4.8/5  (36)
(36)
The compound CH3NH2 reacts with water to form CH3NH3+ and OH-.What role does CH3NH2 play in this reaction?
(Short Answer)
4.9/5  (43)
(43)
What mass of sodium nitrite must be added to 350.mL of water to give a solution with pH = 8.40? [Ka(HNO2)= 5.6 * 10-4]
(Multiple Choice)
4.9/5  (32)
(32)
Identify the conjugate acid-base pairs in the reaction HSO4- + HF 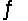 H2SO4 + F-
One conjugate acid-base pair is _______________; the other acid-base pair is ______________.
H2SO4 + F-
One conjugate acid-base pair is _______________; the other acid-base pair is ______________.
(Essay)
4.9/5  (32)
(32)
Calculate the pOH of a solution containing 0.25 g of HCl in 800.mL of solution.
(Short Answer)
4.8/5  (34)
(34)
Consider the weak acid CH3COOH (acetic acid). If a 0.048 M CH3COOH solution is 5.2% ionized, determine the [H3O+] concentration at equilibrium.
(Multiple Choice)
4.8/5  (40)
(40)
The OH- concentration in a 7.5 * 10-3 M Ca(OH)2 solution is
(Multiple Choice)
4.7/5  (37)
(37)
A 0.14 M HNO2 solution is 5.7% ionized. Calculate the H+ ion concentration.
(Multiple Choice)
4.7/5  (34)
(34)
In the reaction H2CO3 + H2O 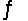 HCO3- + H3O+, the Brønsted acids are
HCO3- + H3O+, the Brønsted acids are
(Multiple Choice)
4.8/5  (32)
(32)
A 8.0 M solution of formic acid (HCOOH)is 0.47% ionized.What is the Ka of formic acid?
(Short Answer)
4.8/5  (40)
(40)
The OH- concentration in a 1.0 * 10-3 M Ba(OH)2 solution is
(Multiple Choice)
4.7/5  (31)
(31)
Calculate the hydrogen ion concentration in a solution of fruit juice having a pH of 4.25.
(Multiple Choice)
4.8/5  (37)
(37)
Showing 61 - 80 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)