Exam 15: Acids and Bases
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
Predict the direction in which the equilibrium will lie for the reaction C6H5COO- + HF 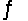 C6H5COOH + F- .
Ka(C6H5COOH)= 6.5 *10-5; Ka(HF)= 7.1 *10-4
C6H5COOH + F- .
Ka(C6H5COOH)= 6.5 *10-5; Ka(HF)= 7.1 *10-4
(Multiple Choice)
4.9/5  (41)
(41)
For maleic acid, HOOCCH=CHCOOH, Ka1 = 1.42 * 10-2 and Ka2 = 8.57 * 10-7. What is the concentration of maleate ion (-OOCCH=CHCOO-)in a 0.150 M aqueous solution of maleic acid?
(Multiple Choice)
4.8/5  (28)
(28)
If the pH of stomach acid is 1.0, what is the hydroxide ion concentration in this solution?
(Short Answer)
4.8/5  (36)
(36)
Showing 161 - 163 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)