Exam 15: Acids and Bases
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
The pOH of a solution is 10.40.Calculate the hydrogen ion concentration in the solution.
(Multiple Choice)
4.8/5  (30)
(30)
Identify the conjugate acid of CO32- in the reaction CO32- + H2PO4- 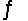 HCO3- + HPO42-
HCO3- + HPO42-
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following yields an acidic solution when dissolved in water?
(Multiple Choice)
4.7/5  (29)
(29)
What is the pH of a solution prepared by mixing 10.0 mL of a strong acid solution with pH = 2.00 and 10.0 mL of a strong acid solution with pH = 6.00?
(Multiple Choice)
5.0/5  (24)
(24)
Hydrosulfuric acid is a diprotic acid, for which Ka1 = 5.7 * 10-8 and Ka2 = 1 *10-19.Determine the concentration of sulfide ion in a 0.10 M hydrosulfuric solution.
(Multiple Choice)
4.8/5  (41)
(41)
Identify the conjugate base of HPO42- in the reaction HCO3- + HPO42- 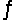 H2CO3 + PO43-
H2CO3 + PO43-
(Multiple Choice)
4.8/5  (38)
(38)
What mass of ammonium chloride must be added to 250.mL of water to give a solution with pH = 4.85? [Kb(NH3)= 1.8 *10-5]
(Multiple Choice)
4.7/5  (38)
(38)
Lime is used in farming to reduce the acidity of the soil. The chemical name for lime is calcium oxide. When water in the soil reacts with lime, what base is formed?
(Short Answer)
4.8/5  (37)
(37)
A 0.10 M NH3 solution is 1.3% ionized. Calculate the H+ ion concentration. NH3 + H2O 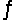 NH4+ + OH-
NH4+ + OH-
(Multiple Choice)
4.9/5  (41)
(41)
In aqueous solutions at 25°C, the sum of the ion concentrations ([H+] + [OH -])equals
1 * 10 - 14.
(True/False)
4.9/5  (37)
(37)
What is the pH of an aqueous solution that contains 5.3 *1017 OH- ions per liter of solution?
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the hydrogen ion concentration in a solution of iced tea with lemon having a pH of 2.87.
(Multiple Choice)
5.0/5  (32)
(32)
The pH of a sample of river water is 6.0. A sample of effluent from a food processing plant has a pH of 4.0. What is the ratio of hydronium ion concentration in the effluent to the hydronium ion concentration in the river?
(Essay)
4.9/5  (36)
(36)
Which one of these salts will form a basic solution upon dissolving in water?
(Multiple Choice)
4.7/5  (43)
(43)
Calculate the pH of a solution containing 0.20 g of NaOH in 2,000.mL of solution.
(Short Answer)
4.9/5  (35)
(35)
Showing 21 - 40 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)