Exam 2: Particles of Matter
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
The density of air at 25°C and 1 atmosphere of pressure is about 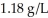 . What happens to the density of air as the temperature is increased? When the pressure is increased?
. What happens to the density of air as the temperature is increased? When the pressure is increased?
(Multiple Choice)
5.0/5  (34)
(34)
Alcohol wiped across a table top rapidly disappears. Which of the following is true?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following units of measurement could be used to describe density?
(Multiple Choice)
4.9/5  (36)
(36)
If the density of mercury is 13.6 g/mL and the density of lead is 11.3 g/mL, which has the larger volume: 1 g of mercury or 1 g of lead?
(Multiple Choice)
4.7/5  (29)
(29)
How would you describe the volume of the following object? the amount of water in a swimming pool
(Multiple Choice)
4.9/5  (34)
(34)
What volume of water would a 52.3-gram sample of pure gold displace? (Assume the density of pure gold equals 19.3 g/mL)
(Multiple Choice)
4.8/5  (36)
(36)
How many milliliters of air are there in a hole measuring 5 L?
(Multiple Choice)
4.9/5  (36)
(36)
The following three boxes represent the number of submicroscopic particles within a given volume of a particular substance at different temperatures. Which box represents the greatest density? Which box represents the greatest temperature? 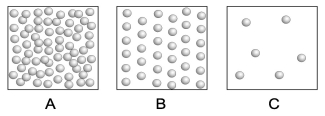
(Multiple Choice)
4.8/5  (41)
(41)
How many calories are there in a candy bar containing 230 Calories?
(Multiple Choice)
4.8/5  (38)
(38)
Why do scuba divers exhale air when they ascend to the surface after a dive?
(Multiple Choice)
5.0/5  (32)
(32)
When a liquid evaporates, the vapor expands because ________.
(Multiple Choice)
4.8/5  (35)
(35)
In which are the molecules moving faster: a swimming pool of boiling water or a cup of boiling water?
(Multiple Choice)
5.0/5  (35)
(35)
When you suck through a soda straw into a drink as shown in the illustration below, what causes the drink to rise into your mouth: the muscles of your lungs and cheeks or the weight of the atmosphere? 
(Multiple Choice)
4.8/5  (40)
(40)
What state (or states)of matter does this diagram of submicroscopic particles represent? 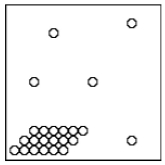
(Multiple Choice)
5.0/5  (43)
(43)
Showing 41 - 60 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)