Exam 2: Particles of Matter
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Which of the following is an example of something with potential energy?
(Multiple Choice)
4.9/5  (31)
(31)
Which has stronger attractions among its submicroscopic particles: a solid at 25°C or a gas at 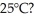
(Multiple Choice)
4.7/5  (30)
(30)
Based on experimental evidence, John Dalton postulated that ________.
(Multiple Choice)
4.7/5  (36)
(36)
Place a card over the open top of a glass filled to the brim with water, and then invert it, as is shown below. Why does the card stay in place? How about when the glass is held sideways? 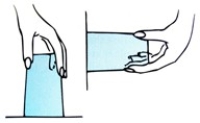
(Multiple Choice)
4.7/5  (35)
(35)
Will your body possess energy after you die? If so, what kind?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following represents the largest quantity of mass?
(Multiple Choice)
4.9/5  (33)
(33)
An old remedy for separating a pair of nested drinking glasses stuck together is to run water at one temperature into the inner glass and then run water at a different temperature over the surface of the outer glass. Which water should be hot and which should be cold?
(Multiple Choice)
4.8/5  (34)
(34)
Each sphere in the diagrams below represents an atom. Joined spheres represent molecules. Assume the two boxes are at the same temperature. Which box contains a higher boiling point liquid? 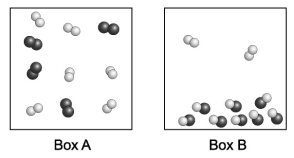
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following is generally true regarding the relationships of the density of an object and its massiveness?
(Multiple Choice)
4.8/5  (36)
(36)
Aristotle described the composition and behavior of matter ________.
(Multiple Choice)
4.9/5  (35)
(35)
When a concentrated acid and fresh water, both at room temperature, are mixed together the result is a solution that is very hot. How does Aristotle's model of matter explain this?
(Multiple Choice)
4.8/5  (25)
(25)
How would you describe the size of the following object? a blood cell
(Multiple Choice)
4.8/5  (35)
(35)
What is the mass in kilograms of a 130-pound human standing on planet Earth?
(Multiple Choice)
4.8/5  (34)
(34)
The scanning probe microscope creates images of atoms by ________.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 21 - 40 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)