Exam 2: Particles of Matter
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
What physical quantities discussed in this chapter change most when a junked car is neatly crushed into a compact cube?
(Multiple Choice)
4.8/5  (32)
(32)
Which has more total energy: a cup of boiling water at 100°C or a swimming pool of slightly cooler water at 90°C?
(Multiple Choice)
4.9/5  (46)
(46)
If the density of a block of ice is 0.92 g/mL, what is the volume of 100. g of ice?
(Multiple Choice)
4.8/5  (38)
(38)
The gravity of the moon is 1/6 that of Earth's. If your mass is 65 kilograms on Earth, what is your mass on the moon?
(Multiple Choice)
4.9/5  (36)
(36)
What happens to the density of a filled water balloon as it is pulled to the bottom of the ocean?
(Multiple Choice)
5.0/5  (35)
(35)
The diagram on the far left shows the moving particles of a gaseous material within a rigid container. Which of the three boxes on the right best represents this material upon the addition of heat? 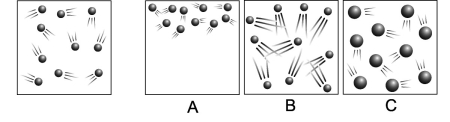
(Multiple Choice)
4.8/5  (30)
(30)
What is the mass in kilograms of a human weighing 130-pounds on the moon?
(Multiple Choice)
4.8/5  (37)
(37)
What would be the effect on an enclosed volume of gas if we were to simultaneously double the pressure and double the Kelvin (Absolute)temperature of the enclosed gas?
(Multiple Choice)
4.9/5  (33)
(33)
The same amount of red colored Kool-Aid crystals are added to a still glass of thick sugar water and a still glass of distilled water. Both are the same temperature. Neither is stirred. Which should become uniform in color first?
(Multiple Choice)
4.8/5  (41)
(41)
A minimum temperature exists (absolute zero). Why does no known maximum temperature exist?
(Multiple Choice)
4.9/5  (37)
(37)
The diagram on the left in box A shows the interface of solid and liquid phases of a single substance where each sphere represents a molecule of that substance. Which box, A, B, or C, best represents what the molecules of this substance would look like if heat were added? Which box best represents what the molecules substance would look like if heat were taken away? 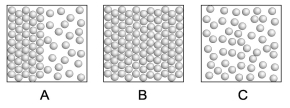
(Multiple Choice)
4.9/5  (34)
(34)
Can an object have mass without having weight? Can it have weight without having mass?
(Multiple Choice)
4.9/5  (38)
(38)
Which would you rather have: a decigram or a kilogram of gold?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is an example of something best described as having kinetic energy?
(Multiple Choice)
4.9/5  (46)
(46)
The phase in which atoms and molecules no longer move is ________.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 61 - 80 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)