Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
How many kilowatt . hours of energy are released from 25 g of deuterium 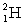 fuel in the fusion reaction:
fuel in the fusion reaction: 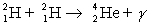 where the masses are
where the masses are 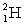 = 2.014 102 u and
= 2.014 102 u and 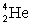 = 4.002 603 u. Notes: Ignore the energy carried off by the gamma ray.
Conversion factors: 1 kWh = 3.600 × 106 J; 1 eV = 1.602 × 10-19 J.
= 4.002 603 u. Notes: Ignore the energy carried off by the gamma ray.
Conversion factors: 1 kWh = 3.600 × 106 J; 1 eV = 1.602 × 10-19 J.
(Multiple Choice)
4.8/5  (38)
(38)
Nucleus A has Z protons and N neutrons. Nucleus B has 2Z protons and 2N neutrons. Nucleus A has a smaller binding energy per nucleon than B. Which entry in the table below is correct? 
(Multiple Choice)
4.7/5  (33)
(33)
Which one of the following particles is not composed of quarks?
(Multiple Choice)
4.9/5  (39)
(39)
A biological tissue is irradiated with neutrons. The biologically equivalent dose of the neutrons is 2.6 × 102 rem. Determine the RBE of the neutrons if the absorbed dose is 130 rd.
(Multiple Choice)
4.8/5  (36)
(36)
Which source of radiation contributes most to the average biological equivalent dose received by a United States resident?
(Multiple Choice)
4.7/5  (32)
(32)
What absorbed dose of protons with an RBE of 17 will cause the same damage to biological tissue as a 200 rd dose of neutrons that have an RBE of 2.6?
(Multiple Choice)
4.8/5  (36)
(36)
Complete the following statement: The average biologically equivalent dose of radiation from consumer products received by a resident of the United States is about
(Multiple Choice)
4.9/5  (35)
(35)
A physicist wishes to measure the exposure of a beam of gamma rays. The beam is passed through 2.00 × 10-2 kg of dry air at STP. The beam produces positive ions in the air which have a total charge of 3.87 × 10-6 C. What is the exposure (in roentgens) of the beam?
(Multiple Choice)
4.9/5  (28)
(28)
A radiologist absorbs 4.0 × 10-5 J of radiation. Determine the absorbed dose if his mass is 74.0 kg.
(Multiple Choice)
4.9/5  (32)
(32)
Consider the nuclear reaction 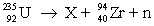 and the masses:
and the masses: 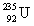 = 235.0439 u;
= 235.0439 u; 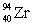 = 93.9063 u; n = 1.008 67 u. If 208.66 MeV of energy is released in this reaction, determine the mass of X.
= 93.9063 u; n = 1.008 67 u. If 208.66 MeV of energy is released in this reaction, determine the mass of X.
(Multiple Choice)
4.7/5  (40)
(40)
Determine the amount of energy released in the following reaction: 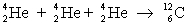 where
where 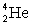 = 4.002 603 u and
= 4.002 603 u and 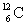 = 12.000 000 u.
= 12.000 000 u.
(Multiple Choice)
4.8/5  (35)
(35)
Astronomers studying the light from calcium atoms located in a galaxy in the constellation Boötes find that the spectral lines are shifted toward the red end of the spectrum. The redshift indicates that the galaxy is moving away at a speed of 3.9 × 106 m/s. What is the distance (in light . years) to the galaxy?
(Multiple Choice)
4.9/5  (39)
(39)
In the induced nuclear reaction, 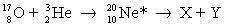 , the reaction produces neon in an excited state, which subsequently decays into a nucleus X and a particle Y. Which one of the following X and Y pairs is not possible?
, the reaction produces neon in an excited state, which subsequently decays into a nucleus X and a particle Y. Which one of the following X and Y pairs is not possible?

(Multiple Choice)
4.8/5  (41)
(41)
Complete the following statement: The term ionizing radiation does not apply to
(Multiple Choice)
4.8/5  (37)
(37)
Determine the atomic number Z and the nucleon number A in the following reaction: 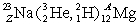 .
. 
(Multiple Choice)
4.8/5  (39)
(39)
In the medical diagnostic technique known as positron emission tomography (PET), a positron and an electron annihilate each other and two -ray photons are emitted. What is the angle between the momentum vectors of the two photons?
(Multiple Choice)
4.7/5  (39)
(39)
What is the importance of thermal neutrons in nuclear processes?
(Multiple Choice)
4.7/5  (38)
(38)
Which one of the following statements is true concerning the reaction 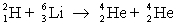 where
where 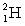 has a mass of 2.014 u;
has a mass of 2.014 u; 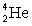 has a mass of 4.003 u;
has a mass of 4.003 u; 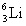 has a mass of 6.015 u; and 1 u = 931.5 MeV?
has a mass of 6.015 u; and 1 u = 931.5 MeV?
(Multiple Choice)
4.8/5  (44)
(44)
Which one of the following statements is the best explanation as to why nuclear fusion is not at present used to generate electric power?
(Multiple Choice)
4.8/5  (45)
(45)
Showing 21 - 40 of 45
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)