Exam 40: Introduction to Quantum Physics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion46 Questions
Exam 17: Sound Waves48 Questions
Exam 18: Superposition and Standing Waves60 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields83 Questions
Exam 24: Gausss Law80 Questions
Exam 25: Electric Potential97 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field105 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 36: Image Formation46 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure50 Questions
Exam 45: Applications of Nuclear Physics40 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
Because of Heisenberg's Uncertainty Principle,the velocity of a particle is known least precisely when the length of the wave packet representing the particle is
(Multiple Choice)
5.0/5  (31)
(31)
The number of photons per second passing a plane perpendicular to a collimated monochromatic (one frequency)beam of light transporting power P is directly proportional to
(Multiple Choice)
4.8/5  (28)
(28)
The light intensity incident on a metallic surface produces photoelectrons with a maximum kinetic energy of 2 eV.The light intensity is doubled.Determine the maximum kinetic energy of the photoelectrons (in eV).
(Multiple Choice)
4.7/5  (31)
(31)
The experimental observation(s)below that require(s)a quantum explanation for the photoelectric effect
(Multiple Choice)
4.9/5  (37)
(37)
Because the factor h on the right side of the Heisenberg uncertainty principle has units of Joule-seconds,it suggests that the energy of a system also has uncertainty.The uncertainty in energy depends on the length of the time interval during which a system exists.ΔEΔt ≥ h/4π.Suppose an unstable mass is produced during a high-energy collision such that the uncertainty in its mass is me/100.(me = 9.11 × 10−31 kg. )How long will this particle exist?
(Multiple Choice)
4.8/5  (36)
(36)
Photoelectrons are ejected when monochromatic light shines on a freshly-prepared (oxide-free)sodium surface.In order to obtain the maximum increase in the number of electrons ejected per second,the experimenter needs to
(Multiple Choice)
4.8/5  (30)
(30)
Assume we can determine the position of a particle within an uncertainty of 0.5 nm.What will be the resulting uncertainty in the particle's momentum (in kg ⋅ m/s)?
(Multiple Choice)
4.8/5  (34)
(34)
What is the shortest x-ray wavelength that can be produced in a 12-keV x-ray machine?
(Short Answer)
4.9/5  (31)
(31)
A pendulum located where 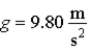 Has a 0.500 kg bob and a 4.00 Hz oscillation frequency.If it is released from a point 0.150 m above the lowest point it reaches,the number of quanta in this oscillation is
Has a 0.500 kg bob and a 4.00 Hz oscillation frequency.If it is released from a point 0.150 m above the lowest point it reaches,the number of quanta in this oscillation is
(Multiple Choice)
4.9/5  (26)
(26)
An atomic level has a lifetime,τ,of 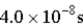 )What is the linewidth,
)What is the linewidth, 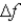 ,of this level?
,of this level?
(Multiple Choice)
4.9/5  (40)
(40)
In electron diffraction,an electron moving at speed v << c acts like
(Multiple Choice)
4.8/5  (41)
(41)
Microscopes are inherently limited by the wavelength of the light used.How much smaller (in order of magnitude)can we "see" using an electron microscope whose electrons have been accelerated through a potential difference of 50000 V than using red light (500 nm)?
(Multiple Choice)
4.9/5  (36)
(36)
A stopping potential of 3.2 V is needed for radiation whose wavelength is 200 nm.What is the work function (in eV)of the material?
(Multiple Choice)
4.7/5  (27)
(27)
Assume electrons are accelerated through a potential difference of 25000 V inside a TV picture tube.What is the minimum wavelength that could be produced when the electrons strike the phosphor? (1 Å = 10−10 m)
(Multiple Choice)
4.7/5  (37)
(37)
When a photon collides with a free electron at rest and the direction of motion of the photon changes,
(Multiple Choice)
4.8/5  (30)
(30)
A neutron has a mass of 1.67 × 10−27 kg.Its de Broglie wavelength is 1.4 × 10−10 m.What is its kinetic energy (in eV)?
(Multiple Choice)
4.9/5  (34)
(34)
Your temperature is 98.6°F.Assuming your skin is a perfect radiator (ε = 1),determine the wavelength corresponding to the largest intensity (in μm).
(Multiple Choice)
4.9/5  (38)
(38)
Showing 21 - 40 of 48
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)