Exam 3: Elements of Chemistry
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Each night you measure your height just before going to bed. When you arise each morning, you measure your height again and consistently find that you are 1 inch taller than you were the night before but only as tall as you were 24 hours ago! Is what happens to your body in this instance best described as a physical change or a chemical change?
(Multiple Choice)
4.9/5  (30)
(30)
The element silicon has a melting point of 1,410°C and a boiling point of 2,355°C. It is a weak conductor of electricity, its density is 2.3 grams per cubic centimeter and it easily forms silicon dioxide when exposed to air. Which of the following is a chemical property of silicon?
(Multiple Choice)
4.7/5  (26)
(26)
Oxygen, 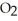 , is certainly good for you. Does it follow that if small amounts of oxygen are good for you then large amounts of oxygen would be especially good for you?
, is certainly good for you. Does it follow that if small amounts of oxygen are good for you then large amounts of oxygen would be especially good for you?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following physical properties would you expect for krypton (Kr)?
(Multiple Choice)
4.9/5  (33)
(33)
A combination of two or more substances in which they no longer retain their chemical properties is called a(n) ________.
(Multiple Choice)
4.8/5  (40)
(40)
The boiling point of methanol is 65°C and the boiling point of ethanol is 78°C. Which of the following statements is true?
(Multiple Choice)
4.8/5  (27)
(27)
Strontium, Sr (number 38), is especially dangerous to humans because it tends to accumulate in calcium-dependent bone marrow tissues (calcium, Ca, number 20). This fact relates to the organization of the periodic table in that strontium and calcium are both ________.
(Multiple Choice)
4.8/5  (37)
(37)
In the winter Vermonters make a tasty treat called "sugar on snow" in which they pour boiled-down maple syrup onto a scoop of clean fresh snow. As the syrup hits the snow it forms a delicious taffy. Which of the following changes are involved in the making of sugar on snow?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following would be considered a physical property?
(Multiple Choice)
4.8/5  (43)
(43)
Some products on the market today involving nanotechnology include ________.
(Multiple Choice)
4.8/5  (39)
(39)
Helium, He, is a nonmetallic gas and the second element in the periodic table. Rather than being placed adjacent to hydrogen, H, however, helium is placed on the far right of the table because ________.
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following would not be considered a chemical property?
(Multiple Choice)
4.9/5  (41)
(41)
The systematic names for water, ammonia, and methane are dihydrogen monoxide, 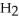 O; trihydrogen nitride, N
O; trihydrogen nitride, N 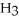 ; and tetrahydrogen carbide, C
; and tetrahydrogen carbide, C 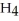 . Why do most people, including chemists, prefer to use the common names for these compounds?
. Why do most people, including chemists, prefer to use the common names for these compounds?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 21 - 40 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)