Exam 3: Elements of Chemistry
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Which of the following elements is a gas at room temperature?
(Multiple Choice)
4.7/5  (33)
(33)
Each circle represents an atom. Which of the following boxes contains an element? A compound? A mixture? 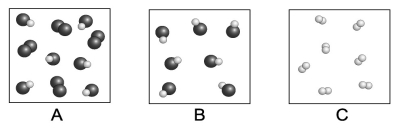
(Multiple Choice)
4.7/5  (35)
(35)
Compared to microtechnology, nanotechnology focuses on a scale that is about ________.
(Multiple Choice)
4.9/5  (30)
(30)
Dioxins are highly toxic compounds that form upon the burning of certain plastics, especially PVC. Dioxins bioaccumulate, which means that animals higher in the food chain tend to have greater concentrations. Most of our exposure to dioxins comes from the food we eat rather than the air we breathe. How many milligrams of dioxins are there in a liter of milk containing 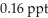 ?
?
(Multiple Choice)
4.9/5  (37)
(37)
The oldest known elements in the periodic table are the ones ________.
(Multiple Choice)
4.8/5  (40)
(40)
If you have two molecules of TiO2, how many oxygen atoms would you have?
(Multiple Choice)
4.9/5  (38)
(38)
How would you classify the following material? coffee (with milk)
(Multiple Choice)
4.9/5  (42)
(42)
If you have one molecule of CO2, how many molecules of O2 does it contain?
(Multiple Choice)
4.9/5  (34)
(34)
Why can't the elements of a compound be separated from one another by physical means?
(Multiple Choice)
4.7/5  (34)
(34)
Mixtures can be separated into their components by taking advantage of differences in the chemical properties of the components. Why might this separation method be less convenient than taking advantage of differences in the physical properties of the components?
(Multiple Choice)
4.9/5  (36)
(36)
Which of these does not describe a metal at room temperature?
(Multiple Choice)
4.8/5  (28)
(28)
Many dry cereals are fortified with iron, which is added to the cereal in the form of small iron particles. How might these particles be separated from the cereal?
(Multiple Choice)
4.8/5  (31)
(31)
What can be said about drinking water that is 99.9999 percent free of some poison, such as a pesticide?
(Multiple Choice)
4.9/5  (38)
(38)
Showing 81 - 100 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)