Exam 8: How Water Behaves
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Why does transforming water from 100°C liquid to 100°C gas require much more energy than to raise the temperature of the same amount of water from absolute zero all the way to 100°C liquid?
(Multiple Choice)
4.7/5  (37)
(37)
Would fevers run higher or lower if water's specific heat were not so high?
(Multiple Choice)
4.8/5  (42)
(42)
What happens to two water molecules after they come together to form a hydrogen bond-do they vibrate more rapidly or less rapidly?
(Multiple Choice)
4.9/5  (42)
(42)
Should the specific heat of your automobile's radiator fluid be as high as possible or as low as possible? Why?
(Multiple Choice)
4.8/5  (35)
(35)
Your instructor hands you a closed flask of room-temperature water. When you hold it, the heat from your bare hands causes the water to boil. Quite impressive! How is this accomplished?
(Multiple Choice)
4.7/5  (43)
(43)
It is important to protect water pipes in your home from freezing because ________.
(Multiple Choice)
4.8/5  (39)
(39)
If all of the liquids pictured above were the same, which setup would contain the capillary with the smallest diameter?
(Multiple Choice)
4.8/5  (36)
(36)
Does putting a lid over a pot of water on a stove shorten the time it takes for the water to come to a boil? After the water is boiling, does use of the lid shorten the cooking time of food?
(Multiple Choice)
4.9/5  (43)
(43)
A water bug can rest on top of water without getting wet because of ________.
(Multiple Choice)
5.0/5  (38)
(38)
Which statement best describes what is happening at the surface of liquid water?
(Multiple Choice)
4.8/5  (38)
(38)
Would you expect the surface tension of water to increase or decrease with temperature?
(Multiple Choice)
4.8/5  (32)
(32)
If water had a lower specific heat, would ponds be more likely to freeze or less likely to freeze?
(Multiple Choice)
4.8/5  (43)
(43)
Why does water not freeze at 0°C when either ions or molecules other than 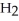 O are present?
O are present?
(Multiple Choice)
4.9/5  (36)
(36)
Why does evaporation of a liquid also cool the gas above it?
(Multiple Choice)
5.0/5  (40)
(40)
Showing 61 - 80 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)