Exam 8: How Water Behaves
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
If the heat of melting of a sample is 135 J/g, what would the heat of freezing for the sample be?
(Multiple Choice)
4.9/5  (43)
(43)
If the specific heat of gold is 0.13 J/(g°C), how much heat does it take to raise the temperature of a 1.0-gram piece of gold from 74°C to 99°C?
(Multiple Choice)
4.9/5  (34)
(34)
What is the temperature change if you cool water from 100°C to 55°C?
(Multiple Choice)
4.7/5  (32)
(32)
Unlike fresh water, ocean water contracts as it is cooled all the way down to its freezing point, which is about -18°C. Why?
(Multiple Choice)
4.9/5  (23)
(23)
Is the density of near-freezing water, which contains microscopic ice crystals, greater or less than the density of liquid water containing no microscopic ice crystals?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements best describes the relationship between temperature and heat?
(Multiple Choice)
4.8/5  (34)
(34)
Why is it that in cold winters a tub of water placed in a farmer's canning cellar helps prevent canned food from freezing?
(Multiple Choice)
4.8/5  (36)
(36)
Suppose that water is used in a thermometer instead of mercury. If the temperature is at 4°C and then changes, why can't the thermometer indicate whether the temperature is rising or falling?
(Multiple Choice)
4.9/5  (40)
(40)
Given the following specific heats and that each sample had the same mass, which of the following materials would cool the slowest?
(Multiple Choice)
4.8/5  (33)
(33)
If an evaporating liquid cools, then does something else warm? If so, what?
(Multiple Choice)
4.8/5  (38)
(38)
Mercury forms a convex meniscus with glass and not the concave meniscus formed by water. What does this tell you about the cohesive forces within mercury versus the adhesive forces between mercury and glass? 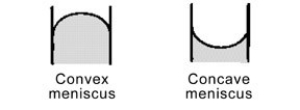
(Multiple Choice)
4.9/5  (35)
(35)
From the diagram above describing the phase of xenon relative to temperature and pressure what is the phase of xenon at 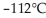 and
and 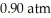 ?
?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the above liquids has the strongest cohesive forces?
(Multiple Choice)
4.8/5  (45)
(45)
If the specific heat of gold is 0.13 J/(g°C), how much heat is needed to raise the temperature of a 20-gram nugget by 20°C?
(Multiple Choice)
4.8/5  (42)
(42)
Bermuda is close to North Carolina, but unlike North Carolina it has a tropical climate year round. Why is this so?
(Multiple Choice)
4.8/5  (38)
(38)
Which graph most appropriately shows the density of water plotted against temperature? 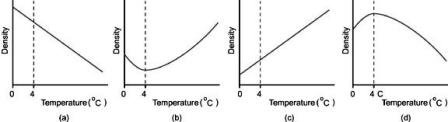
(Multiple Choice)
4.8/5  (40)
(40)
Maple syrup is made from the sap of the sugar maple tree. Why does is take so much energy to make maple syrup?
(Multiple Choice)
4.8/5  (35)
(35)
At the molecular level, when a piece of ice is slowly melting, water molecules are being ________.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 81 - 100 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)