Exam 8: How Water Behaves
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
A great deal of heat is released when liquid water freezes. Why doesn't this heat simply remelt the ice?
(Multiple Choice)
4.8/5  (32)
(32)
Why does liquid water expand slightly when cooled from 4°C to 2°C?
(Multiple Choice)
4.8/5  (27)
(27)
Why does condensation have a tendency to raise the temperature of a liquid?
(Multiple Choice)
4.9/5  (42)
(42)
Ice floats in room temperature water, but does it float in boiling water? Why or why not?
(Multiple Choice)
4.9/5  (35)
(35)
Can a glass be filled to above its brim with water without the water spilling over the edge?
(Multiple Choice)
4.9/5  (32)
(32)
Describe what happens to an ice cube and some water at 0°C if you increase the rate of water formation?
(Multiple Choice)
4.9/5  (28)
(28)
How much heat is required to raise the temperature of 1.00 gram of water from absolute zero, 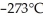 , to
, to 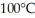 ?
?
(Multiple Choice)
4.8/5  (41)
(41)
If the winds at the latitude of San Francisco and Washington, D.C., were from the east rather than from the west, why might San Francisco be able to grow cherry trees and Washington, D.C. palm trees?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following statements regarding a phase change is NOT true?
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following statements describes what happens when a given mass of a liquid starts to expand?
(Multiple Choice)
4.8/5  (34)
(34)
It easier to cool off on a dry day vs. a humid day because the rate of evaporation is ________.
(Multiple Choice)
4.9/5  (33)
(33)
If you keep a mixture of water and ice at 0°C what will happen?
(Multiple Choice)
4.9/5  (51)
(51)
When you increase the temperature of a substance, the molecules vibrate ________.
(Multiple Choice)
4.8/5  (38)
(38)
The formation of hydrogen bonds between water molecules ________.
(Multiple Choice)
4.8/5  (37)
(37)
From the diagram above describing the phase of water relative to temperature and pressure, is it possible for ice to transform to water vapor (sublime) without ever becoming liquid? At which point on the diagram does this occur?
(Multiple Choice)
4.9/5  (41)
(41)
Showing 121 - 140 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)