Exam 17: Aqueous Ionic Equilibrium
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
Determine the molar solubility of PbSO4 in pure water.Ksp (PbSO4)= 1.82 × 10-8.
(Multiple Choice)
5.0/5  (38)
(38)
Sodium hypochlorite,NaOCl,is the active ingredient in household bleach.What is the concentration of hypochlorite ion if 20.00 mL of bleach requires 32.00 mL of 0.500 M HCl to reach the equivalence point?
(Multiple Choice)
4.9/5  (27)
(27)
A 100.0 mL sample of 0.10 M NH3 is titrated with 0.10 M HNO3.Determine the pH of the solution after the addition of 50.0 mL of HNO3.The Kb of NH3 is 1.8 × 10-5.
(Multiple Choice)
4.8/5  (31)
(31)
How many milliliters of 0.0991 M KOH are required to titrate 25.0 mL of 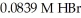 to the equivalence point?
to the equivalence point?
(Multiple Choice)
4.9/5  (39)
(39)
Consider the Ksp values for two compounds: MZ,Ksp = 1.5 × 10-20 and
MZ2,Ksp = 1.5 × 10-20.Why don't these compounds have the same molar solubility?
(Essay)
4.7/5  (37)
(37)
Give the expression for the solubility product constant for SrF2.
(Multiple Choice)
4.8/5  (42)
(42)
What is the pH of a solution made by mixing 30.00 mL of 0.10 M acetic acid with 50.00 mL of 0.100 M KOH? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for 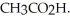
(Multiple Choice)
4.8/5  (35)
(35)
What is the pH of a buffer solution that is 0.255 M in hypochlorous acid (HClO)and 0.333 M in sodium hypochlorite? The Ka of hypochlorous acid is 3.8 × 10-8.
(Multiple Choice)
4.8/5  (43)
(43)
If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NaCHO2 solution is 3.11,which of the following is TRUE?
(Multiple Choice)
4.7/5  (35)
(35)
A buffer solution is 0.100 M in both HC7H5O2 and KC7H5O2 and has a pH of 4.19.Which of the following pH values would you expect from the addition of a small amount of a dilute solution of a strong base?
(Multiple Choice)
4.8/5  (34)
(34)
A solution containing CaCl2 is mixed with a solution of Li2C2O4 to form a solution that is 2.1 × 10-5 M in calcium ion and 4.75 × 10-5 M in oxalate ion.What will happen once these solutions are mixed? Ksp (CaC2O4)= 2.3 × 10-9.
(Multiple Choice)
4.7/5  (41)
(41)
The molar solubility of CuI is 2.26 × 10-6 M in pure water.Calculate the Ksp for CuI.
(Multiple Choice)
4.8/5  (37)
(37)
Describe what happens at neutral pH for aluminum hydroxide.
(Multiple Choice)
4.9/5  (34)
(34)
A 200.0 mL sample of 0.180 M HClO4 is titrated with 0.270 M KOH.Determine the pH of the solution after the addition of 150.0 mL of KOH.
(Multiple Choice)
4.7/5  (33)
(33)
What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN)2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN)2- is 1.0 × 1021.
(Multiple Choice)
4.8/5  (31)
(31)
Showing 121 - 140 of 187
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)