Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
When the following compound is treated with dimethyl sulfide followed by sodium hydride,a product is formed whose formula is C7H12O.Draw a complete,detailed mechanism for this reaction,and predict the structure of the product. 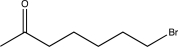
(Short Answer)
4.9/5  (42)
(42)
Which of the following reagents would be the best choice to carry out the reaction shown below? 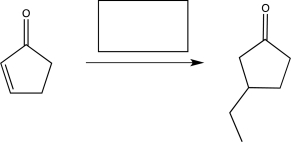
(Multiple Choice)
4.8/5  (38)
(38)
Predict the major product of the following synthetic scheme. 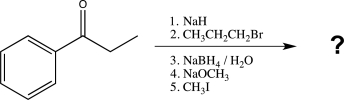
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following functional groups contains a polar π bond with no leaving group?
(Multiple Choice)
4.8/5  (35)
(35)
Could the alkyl halide below be used to generate a sulfonium ylide? Show a mechanism to support your answer. 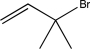
(Essay)
4.8/5  (43)
(43)
Which of the following compounds would you expect to react with a nucleophile at the highest rate? 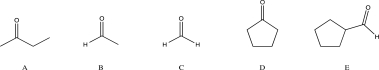
(Multiple Choice)
4.9/5  (41)
(41)
Identify compounds X,Y,and Z in the three-step synthesis shown below. 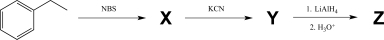
(Short Answer)
4.8/5  (35)
(35)
Predict the major organic product and provide a detailed mechanism for the reaction that would occur in the molecule shown below. 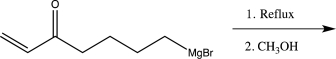
(Short Answer)
4.8/5  (27)
(27)
Predict the major organic product for the following sequence of reactions. 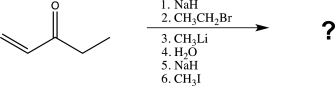
(Short Answer)
4.9/5  (34)
(34)
The chemical behavior of 13C is essentially identical to that of 12C.If the reaction below is carried out,what percentage of the carbon atoms in the product will be 13C? 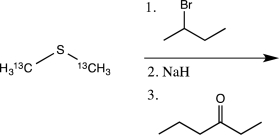
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following molecules would be unlikely to undergo a nucleophilic addition reaction? 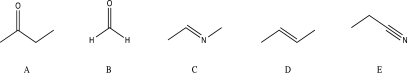
(Multiple Choice)
4.8/5  (29)
(29)
A reaction was carried out,and the two products shown below were obtained.Fill in the boxes with the starting material and the reagents that were used,and then draw a mechanism for the formation of the major product. 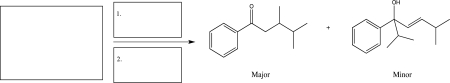
(Short Answer)
4.7/5  (46)
(46)
The Horner-Wadsworth-Emmons reaction is a modification of the Wittig reaction that utilizes a phosphonate-stabilized carbanion to produce an alkene.Propose a detailed mechanism for the Horner-Wadsworth-Emmons reaction shown below. 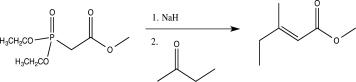
(Short Answer)
4.8/5  (39)
(39)
Two students decide to use different polar aprotic solvents to carry out the reaction below.Student A chooses acetonitrile,while student B chooses tetrahydrofuran.Which is the better choice,and why? 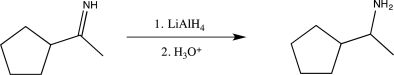
(Essay)
4.7/5  (34)
(34)
Which of the following nucleophiles would tend to favor conjugate addition to an α,β-unsaturated carbonyl?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following compounds would you expect to have the highest percent hydration at equilibrium? 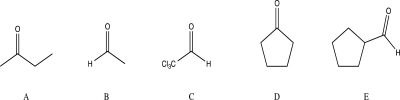
(Multiple Choice)
5.0/5  (38)
(38)
Propose a detailed mechanism for the Knoevenagel condensation,shown below. 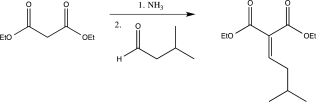
(Short Answer)
4.7/5  (31)
(31)
Which of the following compounds would you expect to react with a nucleophile at the lowest rate? 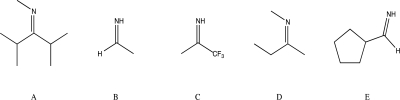
(Multiple Choice)
5.0/5  (35)
(35)
Choose an appropriate set of reagents to carry out the synthetic step shown below. 
(Multiple Choice)
4.9/5  (37)
(37)
Showing 21 - 40 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)