Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Zeros located between two numbers are not significant.
Free
(True/False)
4.9/5  (39)
(39)
Correct Answer:
False
The correct scientific notation for the number 0.00050210 is:
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
D
In addition or subtraction,the result carries the same number of decimal places as the quantity carrying the fewest decimal places.
Free
(True/False)
4.8/5  (33)
(33)
Correct Answer:
True
A conversion factor is a fraction with one unit on top and a different unit on the bottom.
(True/False)
4.9/5  (34)
(34)
How many significant figures should be reported in the answer to the following calculation? 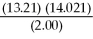 =
=
(Multiple Choice)
4.8/5  (32)
(32)
The correct number of significant figures in the number 865,000 is:
(Multiple Choice)
4.8/5  (32)
(32)
The metric prefix k would be presented as 10 to the power of:
(Multiple Choice)
4.9/5  (42)
(42)
Scientific numbers are reported so that every digit is certain except the last,which is estimated.
(True/False)
5.0/5  (38)
(38)
Given the density of Au is 19.3 g/cm3,determine the mass of gold (in grams)in an ingot with the dimensions of 10.0 in × 4.00 in × 3.00 in.
(Multiple Choice)
4.8/5  (28)
(28)
The correct number of significant figures in the number 0.027090 is:
(Multiple Choice)
4.7/5  (36)
(36)
What is the density of 96 mL of a liquid that has a mass of 90.5 g?
(Multiple Choice)
4.8/5  (41)
(41)
Numbers are usually written so that the uncertainty is in the last reported digit.
(True/False)
4.8/5  (38)
(38)
Showing 1 - 20 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)