Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
A lead ball has a mass of 55.0 grams and a density of 11.4 g/cm3.What is the volume of the ball?
(Multiple Choice)
4.9/5  (35)
(35)
Determine the answer to the following equation with correct number of significant figures:
(17.103 + 2.03)× 1.02521 = ________
(Multiple Choice)
4.9/5  (35)
(35)
A popular science demonstration is to take several liquids that will not mix together and "stack" these liquids in a tall glass cylinder.Suppose the following three liquids were placed in the same tall,narrow glass cylinder: 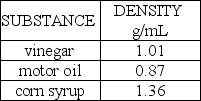 These liquids would stack in which order?
These liquids would stack in which order?
(Multiple Choice)
4.8/5  (40)
(40)
When the number 2.35 is rounded to contain 2 significant figures it becomes 2.4.
(True/False)
4.9/5  (35)
(35)
What is the volume of 19.6 g of a liquid that has a density of 0.967 g/mL?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following sets of units is NOT in the order of increasing size?
(Multiple Choice)
4.9/5  (35)
(35)
Trailing zeros before a decimal point but after a non-zero number are considered significant figures.
(True/False)
4.8/5  (35)
(35)
How many significant figures are represented by the following number that is written in scientific notation? 2.5 × 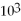
(Multiple Choice)
4.7/5  (31)
(31)
The jet fuel in an airplane has a mass of 97.5 kg and a density of 0.804 g/cm3.What is the volume of this jet fuel?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following statements is NOT part of the rules for determining significant figures?
(Multiple Choice)
4.9/5  (41)
(41)
In multiplication or division calculations,the answer will have the same number of decimal places as the number carrying the most decimal places.
(True/False)
4.9/5  (36)
(36)
When rounding the number 2.348615 to 4 significant figures,what is the correct value?
(Multiple Choice)
4.7/5  (42)
(42)
The distance from New York City to Washington,DC is approximately 235 miles.Identify the correct solution map to convert from miles to kilometers using the prefix multipliers and the given conversion factors: 1 mile = 5280 ft;1 ft = 12 in;1 in = 2.54 cm.
(Multiple Choice)
4.7/5  (33)
(33)
A room has dimensions of 10.0 ft × 20.0 ft × 8.00 ft.Given that there are three feet in a yard,what is the volume of the room in yd3?
(Multiple Choice)
4.9/5  (29)
(29)
The prefix in the name of a polygon indicates how many sides this geometric figure has,so a "decagon" would have ten sides.
(True/False)
5.0/5  (45)
(45)
The typical problem-solving procedure involves four steps in the order:
(Multiple Choice)
4.9/5  (42)
(42)
Showing 81 - 100 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)