Exam 16: Oxidation and Reduction
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
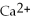 (aq)+ 2Na (s)→ Ca (s)+
(aq)+ 2Na (s)→ Ca (s)+ 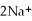 (aq)is a spontaneous reaction.
Activity Series =
(aq)is a spontaneous reaction.
Activity Series = 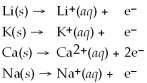
Free
(True/False)
4.8/5  (45)
(45)
Correct Answer:
False
The oxidation number of a chlorine atom is -1.
Free
(True/False)
4.8/5  (34)
(34)
Correct Answer:
False
Using the activity list included in this problem,which element/ion is the most resistant to oxidation?
Activity Series = 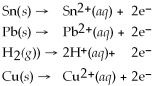
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
C
Identify the substance being reduced in the following reaction: 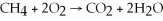 .
.
(Multiple Choice)
4.8/5  (42)
(42)
If you properly balance the following half reaction in base solution,how many electrons would appear on the product side of the equation? Al (s)→ Al(OH)41- (aq)
(Multiple Choice)
4.8/5  (38)
(38)
Which substance below would contain a nitrogen atom with the highest oxidation number of all those shown?
(Multiple Choice)
4.8/5  (37)
(37)
A spontaneous redox reaction can be used to produce electrical current.
(True/False)
5.0/5  (37)
(37)
Which of the following are typically TRUE of a reducing agent?
1.It causes reduction.
2.It gains electron(s).
3.It is the oxidized substance.
(Multiple Choice)
4.8/5  (31)
(31)
What is the oxidation state of the underlined atom in the reaction: 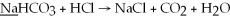
(Multiple Choice)
4.8/5  (34)
(34)
From the activity list included in this problem,which element/ion is the most difficult to reduce?
Activity Series = 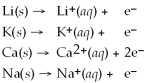
(Multiple Choice)
4.9/5  (36)
(36)
In the following reaction,
Zn (s)+ CuSO4 (aq)→ ZnSO4 (aq)+ Cu (s):
(Multiple Choice)
4.9/5  (38)
(38)
It can be shown that copper metal lies below H2 on the activity series of metals.This means that copper will dissolve in hydrochloric acid.
(True/False)
5.0/5  (40)
(40)
From the activity list included in this problem,which element/ion will spontaneously react with 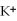 ?
Activity Series =
?
Activity Series = 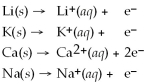
(Multiple Choice)
4.7/5  (41)
(41)
Electrical current can be used to drive a nonspontaneous redox reaction.
(True/False)
4.9/5  (35)
(35)
Electrons flowing through a wire is an example of electrical current.
(True/False)
4.8/5  (33)
(33)
Showing 1 - 20 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)