Exam 12: Liquids,solids,and Intermolecular Forces
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
The ability of sodium chloride to mix with water is most likely due to:
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
B
Attraction between the Na+ cation and Cl- anion holds the solid lattice of sodium chloride together.
(True/False)
4.7/5  (33)
(33)
Sublimation is the process of a liquid being converted directly to a gas.
(True/False)
4.7/5  (40)
(40)
If we supply additional heat to a solid in equilibrium with its liquid at the melting point,the thermal energy added is used to:
(Multiple Choice)
4.8/5  (45)
(45)
Which intermolecular force is common to all polar molecules but NOT nonpolar molecules?
(Multiple Choice)
4.7/5  (36)
(36)
Rank the compounds NH3,CH4,and PH3 in order of increasing boiling point.
(Multiple Choice)
4.9/5  (35)
(35)
Which substance would be expected to have the highest boiling point?
(Multiple Choice)
4.9/5  (34)
(34)
Increasing the temperature increases the vaporization rate of a liquid because the excess energy is used to break covalent bonds.
(True/False)
4.8/5  (42)
(42)
Ice can float in a glass of liquid water because the solid form of water is more dense than the liquid form.
(True/False)
4.9/5  (41)
(41)
Water has unusual physical properties which enable life to exist on earth.
(True/False)
4.8/5  (37)
(37)
How many kJ of heat are needed to completely melt 1.70 moles of 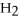 O,given that the water is at its melting point? The heat of fusion for water is
O,given that the water is at its melting point? The heat of fusion for water is 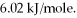
(Multiple Choice)
4.9/5  (34)
(34)
The measure of the resistance to the flow of a liquid is called:
(Multiple Choice)
4.7/5  (40)
(40)
Viscosity increases with increased intermolecular force because the molecules attract each other strongly which hinders the flow.
(True/False)
4.7/5  (33)
(33)
Showing 1 - 20 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)