Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
A 12-oz can of soda pop costs eighty-nine cents.A 2.00 L bottle of the same variety of soda pop costs $2.29.How many times more expensive it is to buy the 12-oz can of pop compared to buying it in a 2.00 L bottle? (1.00 L = 1.057 quart and 1 quart contains 32 oz)
(Multiple Choice)
4.9/5  (29)
(29)
The mass of an object,4.55 × 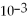 g,expressed in decimal notation is 0.000455 g.
g,expressed in decimal notation is 0.000455 g.
(True/False)
4.8/5  (38)
(38)
A solution map is the section near the back of the textbook that provides the answers to assigned problems.
(True/False)
4.8/5  (28)
(28)
How many significant figures should be reported in the answer to the following calculation?
(43.980)× (19.0023 + 25)=
(Multiple Choice)
4.8/5  (38)
(38)
Given the following list of densities,which materials would float in a molten vat of lead provided that they do not themselves melt? Densities (g/mL): lead = 11.4,glass = 2.6,gold = 19.3,charcoal = 0.57,platinum = 21.4.
(Multiple Choice)
4.8/5  (37)
(37)
Showing 121 - 131 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)