Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
When the temperature of an object is reported as 23.7°C,the actual temperature can be assumed to be between 23.6°C and 23.8°C.
(True/False)
4.9/5  (42)
(42)
How many significant figures should be reported in the answer to the following calculation?
(4.921)+ (16.2)=
(Multiple Choice)
4.9/5  (42)
(42)
The correct number of significant figures in the number 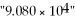 Is:
Is:
(Multiple Choice)
4.9/5  (34)
(34)
Determine the answer for the equation below with correct number of significant figures:
1.2 × 1.79 = ________
(Multiple Choice)
4.9/5  (35)
(35)
The distance between the two hydrogen atoms in a molecule of water is 0.000000000172 m.Express this distance in scientific notation.
(Multiple Choice)
4.8/5  (39)
(39)
Determine the answer for the equation below with correct number of significant figures:
3.215 × 13.2 ÷ 0.218 = ________
(Multiple Choice)
4.8/5  (37)
(37)
Metals expand to a larger volume when heated.If a piece of metal was heated,which one of the following statements would be TRUE?
(Multiple Choice)
4.9/5  (37)
(37)
How many low dose 81 mg aspirin tablets can be made from 1.21 kg of aspirin?
(Multiple Choice)
4.7/5  (38)
(38)
Determine the answer to the following equation with correct number of significant figures:
(4.123 × 0.12)+ 24.2 = ________
(Multiple Choice)
4.8/5  (39)
(39)
How many significant figures should be reported in the answer to the following calculation?
(1.40)× (17.1)=
(Multiple Choice)
4.9/5  (28)
(28)
Trailing zeros at the end of a number,but before an implied decimal point,are ambiguous.
(True/False)
4.8/5  (38)
(38)
Showing 21 - 40 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)