Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
The correct number of significant figures in the number 4.0 × 10-2 is:
(Multiple Choice)
4.9/5  (36)
(36)
Zeros located after a number and after a decimal point are significant.
(True/False)
4.8/5  (34)
(34)
You do not need to write units in calculations as long as you can remember them.
(True/False)
4.8/5  (32)
(32)
The Olympic Games shot put field event uses a 16 pound (lb)shot.Identify the correct solution map to convert from pounds to kilograms using prefix multipliers and the given conversions of 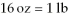 And
And 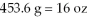 .
.
(Multiple Choice)
5.0/5  (35)
(35)
Suppose a thermometer has marks at every one degree increment and the mercury level on the thermometer is exactly between the 25 and 26 degree Celsius marks.We should properly report the temperature measurement as:
(Multiple Choice)
4.8/5  (35)
(35)
How many significant digits should be reported in the answer to the following calculation?
(4.3 - 3.7)× 12.3 =
(Multiple Choice)
4.8/5  (37)
(37)
The correct number of significant figures in the number 1.250100 is:
(Multiple Choice)
4.8/5  (35)
(35)
An American nickel five cent coin has a mass of approximately 5 grams.Five grams is equivalent to which term?
(Multiple Choice)
4.9/5  (37)
(37)
In multiplication and division calculations,the answer will have the same number of decimal places as the number carrying the fewest decimal places.
(True/False)
4.8/5  (35)
(35)
The density of iron is 7.86 g/cm3 while the density of lead is 11.4 g/cm3.If you had one cm3 of each metal,the piece of iron would have a greater mass.
(True/False)
4.8/5  (40)
(40)
What is the density (g/mL)of an object that has a mass of 14.01 grams and,when placed into a graduated cylinder,causes the water level to rise from 25.2 mL to 33.6 mL?
(Multiple Choice)
4.8/5  (48)
(48)
Which of the following would NOT be considered a correct conversion factor?
(Multiple Choice)
4.8/5  (25)
(25)
Determine the answer to the following equation with correct number of significant figures:
106 ÷ 9.02 × 1.9 = ________
(Multiple Choice)
4.8/5  (35)
(35)
Showing 101 - 120 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)