Exam 12: Liquids,solids,and Intermolecular Forces
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
How many joules of heat are needed to completely vaporize 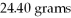 Of water at its boiling point?
Given
Of water at its boiling point?
Given 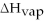 = 40.6 kJ/mol
= 40.6 kJ/mol
(Multiple Choice)
4.9/5  (39)
(39)
Paradichlorobenzene,a material used in "moth balls," is known to go directly from a solid form to a gaseous form.This process is known as:
(Multiple Choice)
4.8/5  (40)
(40)
How many grams of 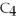
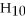 O can be melted by
O can be melted by 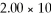 3 J?
Given
3 J?
Given 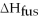 =7.27 kJ/mol
=7.27 kJ/mol
(Multiple Choice)
4.8/5  (37)
(37)
Which state of matter has a high density and an indefinite shape?
(Multiple Choice)
4.8/5  (36)
(36)
Which state of matter has a high density and a definite volume?
(Multiple Choice)
4.8/5  (45)
(45)
Oil would be considered a nonvolatile liquid while gasoline would be considered volatile.
(True/False)
4.9/5  (34)
(34)
Evaporation is decreased by increasing the intermolecular forces.
(True/False)
4.9/5  (41)
(41)
In northern climates,it is common to have a layer of frost form on cars that have been out overnight in the winter.During the day the frost layer disappears despite the temperature of the ice remaining below freezing.How?
(Multiple Choice)
4.8/5  (34)
(34)
A glass of ice water containing nine ice cubes will be colder than a similar-size glass of ice water containing only three ice cubes.
(True/False)
4.8/5  (31)
(31)
Without intermolecular forces,solids and liquids would not exist and all matter would be gaseous.
(True/False)
4.9/5  (54)
(54)
The process of heating a pot of water from room temperature to boiling temperature is an exothermic process.
(True/False)
4.9/5  (36)
(36)
Liquids that have high vapor pressure and low boiling points are called:
(Multiple Choice)
4.8/5  (43)
(43)
Which intermolecular force increases with increasing molar mass?
(Multiple Choice)
4.8/5  (35)
(35)
Which statement is TRUE in describing what occurs when a solid melts to a liquid?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 41 - 60 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)