Exam 12: Liquids,solids,and Intermolecular Forces
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Solids usually have much greater densities than gases because molecules of a solid are much farther apart.
(True/False)
4.9/5  (39)
(39)
Intermolecular forces are the attractive forces between atoms within a compound.
(True/False)
4.9/5  (41)
(41)
All intermolecular forces are broken when a liquid vaporizes into a gas.
(True/False)
4.7/5  (41)
(41)
Compare a small pot of water that is boiling vigorously to a large pot of water that is boiling gently.Which statement is TRUE?
(Multiple Choice)
5.0/5  (40)
(40)
Compounds with very high vapor pressures must have very minimal intermolecular forces.
(True/False)
4.8/5  (42)
(42)
How many kJ of heat are needed to completely vaporize 23.4 g of 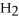 O? The heat of vaporization for water at the boiling point is
O? The heat of vaporization for water at the boiling point is 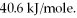
(Multiple Choice)
4.7/5  (36)
(36)
Which state of matter has a low density and an indefinite volume?
(Multiple Choice)
4.8/5  (35)
(35)
Water has a heat of vaporization of 44.0 kJ/mol while the chemical diethyl ether has a heat of vaporization of 27.1 kJ/mol.This shows that one mole of water would vaporize more easily than would one mole of diethyl ether.
(True/False)
4.8/5  (46)
(46)
How many kJ of heat are needed to completely vaporize 3.30 moles of 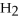 O? The heat of vaporization for water at the boiling point is
O? The heat of vaporization for water at the boiling point is 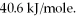
(Multiple Choice)
4.7/5  (35)
(35)
Substance A is a molecular compound that dissolves in gasoline but not in water.The molecules of A are very likely:
(Multiple Choice)
4.9/5  (41)
(41)
The shapes of protein molecules are determined by intermolecular forces.
(True/False)
4.8/5  (33)
(33)
The amount of heat required to melt one mole of a solid is called the:
(Multiple Choice)
4.8/5  (25)
(25)
Dispersion forces result from the temporary distortion of the electron cloud in an atom or molecule which increases in magnitude with increasing size.
(True/False)
4.7/5  (34)
(34)
Showing 61 - 80 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)