Exam 12: Liquids,solids,and Intermolecular Forces
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Ion-dipole forces occur in mixtures of ionic compounds and polar compounds.
(True/False)
4.9/5  (40)
(40)
Which sequence correctly shows the increasing density of the three phases of water?
(Multiple Choice)
4.9/5  (32)
(32)
The rate of vaporization of a liquid can be increased by:
1.increasing the surface area
2.increasing the temperature
3.increasing the strength of the intermolecular forces
(Multiple Choice)
4.8/5  (42)
(42)
For hydrogen bonding to occur,a molecule must have a hydrogen atom bonded directly to a fluorine,oxygen,or nitrogen atom.
(True/False)
4.7/5  (43)
(43)
Gaseous water vapor can frost the windows of a car on a cold morning.This process of a gas changing directly into a solid is known as:
(Multiple Choice)
4.8/5  (31)
(31)
Which state of matter has a low density and is easily compressed?
(Multiple Choice)
4.8/5  (29)
(29)
Intermolecular forces hold atoms and molecules in place in a solid.
(True/False)
4.9/5  (34)
(34)
Intermolecular forces determine if a substance is a solid,liquid or gas at room temperature.
(True/False)
4.8/5  (37)
(37)
The heat of fusion for water is significantly more than the heat of vaporization for water because fusion requires complete separation of one molecule from another.
(True/False)
4.9/5  (37)
(37)
What is the heat of vaporization(kJ/mol)if it takes 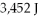 Of heat to completely vaporize
Of heat to completely vaporize 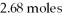 Of the liquid at its boiling point?
Of the liquid at its boiling point?
(Multiple Choice)
4.8/5  (43)
(43)
When one mole of water vapor at 100°C condenses,it will release an amount of energy equivalent to its heat of vaporization (40.7 kJ).
(True/False)
4.7/5  (42)
(42)
H2 boils at a higher temperature than He because H2 undergoes hydrogen bonding.
(True/False)
4.8/5  (33)
(33)
Showing 81 - 100 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)