Exam 12: Liquids,solids,and Intermolecular Forces
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Which intermolecular force is due to the formation of an instantaneous dipole?
(Multiple Choice)
4.8/5  (38)
(38)
The rate of evaporation will increase if you pour a liquid out onto a large surface.
(True/False)
4.9/5  (41)
(41)
When sufficient quantity of heat has been added to reach the boiling point of a solution,what happens to any additional heat added?
(Multiple Choice)
5.0/5  (36)
(36)
Atomic solids,such as graphite,have a weak dispersion force holding them together.
(True/False)
4.8/5  (29)
(29)
The melting point is reached when sufficient energy has been added to the molecules in a substance to overcome the intermolecular forces holding them stationary.
(True/False)
4.8/5  (41)
(41)
Increasing the intermolecular forces of a liquid will do which of the following?
(Multiple Choice)
4.8/5  (39)
(39)
Compounds that are similar to water in molecular mass all exist as solids.
(True/False)
4.8/5  (38)
(38)
Liquids that are viscous flow more slowly than liquids that are not viscous.
(True/False)
4.9/5  (37)
(37)
How many kJ of heat are needed to completely melt 95.3 g of copper metal,given that the metal is at its melting point? The heat of fusion for this metal is 13.1 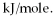
(Multiple Choice)
4.9/5  (41)
(41)
Showing 101 - 114 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)