Exam 16: Oxidation and Reduction
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
For the reaction KMnO4 + Li → LiMnO4 + K,which atom is being reduced?
(Multiple Choice)
4.9/5  (33)
(33)
What is the oxidation state of the underlined atom in the reaction: 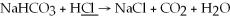
(Multiple Choice)
4.8/5  (32)
(32)
In an electrochemical cell,which of the following statements is FALSE?
(Multiple Choice)
4.9/5  (38)
(38)
From the activity list included in this problem,which element will displace  Ions from solution?
Activity Series =
Ions from solution?
Activity Series = 
(Multiple Choice)
4.7/5  (48)
(48)
From the activity list included in this problem,which element/ion is the most difficult to oxidize?
Activity Series = 
(Multiple Choice)
4.9/5  (37)
(37)
The driving force that causes electrons to flow through a wire is called current.
(True/False)
4.8/5  (42)
(42)
Reactions involving the transfer of electrons are called redox reactions.
(True/False)
4.7/5  (39)
(39)
The half-reaction 2 Br 1- → Br2 + 2 e- is balanced with respect to mass but it is not balanced with respect to charge.
(True/False)
4.8/5  (45)
(45)
From the activity list included in this problem,which element/ion is the easiest to reduce?
Activity Series = 
(Multiple Choice)
5.0/5  (44)
(44)
A sacrificial electrode works by being reduced which prevents the metal from being oxidized.
(True/False)
5.0/5  (47)
(47)
From the activity list included in this problem,which element/ion is the easiest to reduce?
Activity Series = 
(Multiple Choice)
4.9/5  (35)
(35)
To achieve the largest battery voltage possible,the battery should be assembled from a metal high on the activity series list with a metal ion low on the activity series list.
(True/False)
4.9/5  (49)
(49)
Which of the following are typically TRUE of an oxidizing agent?
1.It causes oxidation.
2.It gains electron(s).
3.It is the reduced substance.
(Multiple Choice)
4.9/5  (40)
(40)
Cars are being developed that run on hydrogen gas and the only emission is water.
(True/False)
4.7/5  (39)
(39)
Distinguish between a galvanic (voltaic)cell and an electrolytic cell.
(Essay)
4.9/5  (31)
(31)
An electrochemical cell is based on the coupling of a spontaneous redox reaction.
(True/False)
4.9/5  (32)
(32)
Showing 21 - 40 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)