Exam 16: Oxidation and Reduction
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Electrolysis is used to recover many metals from their oxide ores.
(True/False)
4.9/5  (35)
(35)
What is the oxidation state of the underlined atom in the compound: C 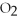 ?
?
(Multiple Choice)
4.9/5  (43)
(43)
What is the balanced oxidation half-reaction for the following unbalanced redox reaction? 
(Multiple Choice)
4.9/5  (41)
(41)
For the reaction Si (s)+ O2 (g)→ SiO2 (g),the reducing agent is:
(Multiple Choice)
4.9/5  (34)
(34)
What is the oxidation state of the underlined atom in the compound: 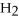 S
S 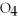 ?
?
(Multiple Choice)
4.7/5  (42)
(42)
What is the oxidation state of the underlined atom in the compound: 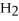 S
S 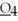 ?
?
(Multiple Choice)
4.8/5  (45)
(45)
Reduction involves which of the following?
1.Loss of electron(s).
2.Gain of electron(s).
3.Decrease in oxidation state.
(Multiple Choice)
4.8/5  (40)
(40)
For the reaction Co + Cl2 → CoCl2 ,which statement is TRUE?
(Multiple Choice)
4.9/5  (41)
(41)
Oxidation involves which of the following?
1.Loss of electron(s).
2.Gain of electron(s).
3.Increase in oxidation state.
(Multiple Choice)
4.8/5  (40)
(40)
The reaction 2 NO2 → N2O4 would not be considered a redox reaction.
(True/False)
4.8/5  (42)
(42)
Showing 61 - 80 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)