Exam 16: Oxidation and Reduction
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
What is the oxidation state of the underlined atom in the reaction: 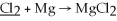
(Multiple Choice)
4.8/5  (31)
(31)
Balance the following half reaction in base solution: H20 (l)→ H2 (g)
(Multiple Choice)
4.8/5  (45)
(45)
Most acids dissolve metals by the oxidation of H+ ions to hydrogen gas.
(True/False)
4.8/5  (33)
(33)
Oxidation and reduction cannot both occur in the same chemical reaction.
(True/False)
4.9/5  (42)
(42)
Corrosion of metals may be prevented by keeping them dry at all times.
(True/False)
4.9/5  (32)
(32)
What must be done to the following half-reactions before they can be added together?
Mn (s)→ Mn 2+(aq)+ 2e-
Fe 3+ (aq)+ 3 e- → Fe (s)
(Multiple Choice)
4.9/5  (36)
(36)
How many electrons are exchanged when the following reaction is balanced?
Zn (s)+ Ni 3+ (aq)→ Zn 2+ (aq)+ Ni (s)
(Multiple Choice)
4.8/5  (34)
(34)
Assign the oxidation state of each atom in sodium sulfate,Na2SO4.
(Multiple Choice)
4.8/5  (44)
(44)
Using the activity list included in this problem,which element/ion is the most resistant to reduction?
Activity Series = 
(Multiple Choice)
4.8/5  (41)
(41)
From the activity list included in this problem,which element will react with Cu?
Activity Series = 
(Multiple Choice)
4.8/5  (34)
(34)
What is the oxidation state of the underlined atom in the reaction: 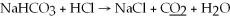
(Multiple Choice)
4.9/5  (38)
(38)
From the activity list included in this problem,which element will not dissolve in dilute hydrochloric acid?
Activity Series = 
(Multiple Choice)
4.9/5  (36)
(36)
From the activity list included in this problem,which element/ion is the most easily oxidized?
Activity Series = 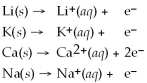
(Multiple Choice)
4.9/5  (31)
(31)
Showing 41 - 60 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)