Exam 16: Oxidation and Reduction
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases122 Questions
Exam 12: Liquids,solids,and Intermolecular Forces114 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry119 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Using the activity list included in this problem,which element/ion listed below is the most easily oxidized?
Activity Series = 
(Multiple Choice)
4.8/5  (35)
(35)
From the activity list included in this problem,which element below would serve as a sacrificial electrode for iron?
Activity Series = 
(Multiple Choice)
4.9/5  (38)
(38)
The rusting of iron is an example of the process known as corrosion.
(True/False)
4.8/5  (39)
(39)
Identify the substance being oxidized in the following reaction: 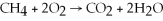 .
.
(Multiple Choice)
4.8/5  (39)
(39)
Assign the oxidation state of each atom in potassium chlorate,KClO3.
(Multiple Choice)
4.7/5  (36)
(36)
In an electrochemical cell,which of the following statements is FALSE?
(Multiple Choice)
4.9/5  (38)
(38)
Given that the Activity Series shown below is accurate,then Li(s)+ 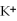 (aq)→
(aq)→ 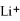 (aq)+ K(s)is a spontaneous reaction.
Activity Series =
(aq)+ K(s)is a spontaneous reaction.
Activity Series = 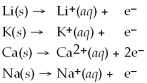
(True/False)
4.8/5  (34)
(34)
Oxidation can be defined as the gain of oxygen atoms by another element.
(True/False)
4.7/5  (36)
(36)
What is the balanced reduction half-reaction for the following unbalanced redox reaction: 
(Multiple Choice)
4.7/5  (34)
(34)
What is the balanced oxidation half-reaction for the following unbalanced redox reaction: 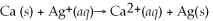
(Multiple Choice)
4.8/5  (31)
(31)
Suggest two methods to reduce corrosion of a metal such as iron and briefly explain how each method works.
(Essay)
4.9/5  (35)
(35)
Reduction can be defined as the gain of oxygen atoms by another element.
(True/False)
4.8/5  (46)
(46)
Showing 81 - 100 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)