Exam 14: Wave Particle Duality and Quantum Physics
Exam 1: The Electric Field I: Discrete Charge Distributions87 Questions
Exam 2: The Electric Field II: Continuous Charge Distributions75 Questions
Exam 3: Electric Potential108 Questions
Exam 4: Capacitance73 Questions
Exam 5: Electric Current and Direct-Current Circuits160 Questions
Exam 6: The Magnetic Field71 Questions
Exam 7: Sources of the Magnetic Field115 Questions
Exam 8: Magnetic Induction84 Questions
Exam 9: Alternating-Current Circuits119 Questions
Exam 10: Maxwells Equations and Electromagnetic Waves61 Questions
Exam 11: Properties of Light116 Questions
Exam 12: Optical Images143 Questions
Exam 13: Interference and Diffraction116 Questions
Exam 14: Wave Particle Duality and Quantum Physics153 Questions
Exam 15: Applications of the Schrodinger Equation54 Questions
Exam 16: Atoms128 Questions
Exam 17: Molecules44 Questions
Exam 18: Solids and the Theory of Conduction83 Questions
Exam 19: Relativity83 Questions
Exam 20: Nuclear Physics135 Questions
Exam 21: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
The visible portion of the electromagnetic spectrum extends from
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the photon energy for light of wavelength 600 nm. (Planck's constant h = 6.626 *10-34 J·s)
(Multiple Choice)
4.7/5  (36)
(36)
An electron is confined to a region of space of length L = 0.2 nm. Its minimum kinetic energy, assuming that p = p, is approximately
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following particles has the longest wavelength?
(Multiple Choice)
5.0/5  (44)
(44)
A proton is in a one-dimensional box of length 2 *10-15 m. Find the wavelength of the photon emitted for a transition between the first excited state and the ground-state.
(Multiple Choice)
4.8/5  (41)
(41)
For an electron to have a de Broglie wavelength of 0.10 nm it must be accelerated through a potential difference of
(Multiple Choice)
4.7/5  (31)
(31)
The photoelectric threshold of a certain metal is 310 nm. The maximum kinetic energy of electrons ejected from the surface of the metal by ultraviolet light of wavelength
200 nm is
(Multiple Choice)
4.7/5  (49)
(49)
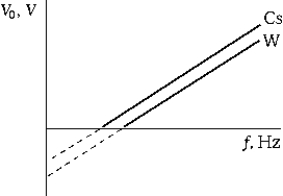 The graph shows the stopping potential for photoelectrons as a function of the frequency of the incident light for two different metals (Cs and W). Which of the following statements is true?
The graph shows the stopping potential for photoelectrons as a function of the frequency of the incident light for two different metals (Cs and W). Which of the following statements is true?
(Multiple Choice)
4.8/5  (32)
(32)
A classical point particle moves back and forth with constant speed between two walls at x = 0 and x = 6 cm. What is the probability of finding the particle between x = 4.0 cm and x = 4.4 cm?
(Multiple Choice)
4.9/5  (40)
(40)
Suppose an electron is confined in a one-dimensional box of width L which is two times the Bohr's radius of 0.0529 nm. The energy of the ground state of the electron is
(Multiple Choice)
4.8/5  (36)
(36)
The wavelength of an electron is 1.33 nm. Its kinetic energy is approximately
(Multiple Choice)
4.8/5  (35)
(35)
A 10.0-kg mass moving with a velocity of 100 m/s has a de Broglie wavelength of approximately
(Multiple Choice)
4.9/5  (43)
(43)
A mass of 0.250 kg is oscillating with small amplitude oscillations on the end of a spring whose stiffness constant is 10 N/m. When this oscillator makes a transition from its n = 6 state to its n = 5 state, the energy of the photon emitted is
(Multiple Choice)
4.9/5  (36)
(36)
Potassium has a work function of 2.3 eV for photoelectric emission. Which of the following wavelengths is the longest wavelength for which photoemission occurs?
(Multiple Choice)
4.9/5  (33)
(33)
A particle is confined in a one-dimensional box. The ground-state energy is E. If a second particle of twice the mass is placed in a second box of twice the length, its ground-state energy is
(Multiple Choice)
4.9/5  (39)
(39)
Photons of wavelength 0.00150 nm undergo Compton collisions with free electrons. What is the energy of the scattered photons whose angle of scattering is 45º?
(Multiple Choice)
4.8/5  (42)
(42)
A classical point particle moves back and forth with constant speed between two walls at x = 0 and x = 4 cm. What is the probability of finding the particle between x = 3 cm and x = 3.4 cm?
(Multiple Choice)
4.7/5  (33)
(33)
Microwaves range in wavelength from about 2.0 * 10-2 cm to about 5.0 cm. What is the maximum energy in eV that a microwave may have?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 81 - 100 of 153
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)