Exam 14: Wave Particle Duality and Quantum Physics
Exam 1: The Electric Field I: Discrete Charge Distributions87 Questions
Exam 2: The Electric Field II: Continuous Charge Distributions75 Questions
Exam 3: Electric Potential108 Questions
Exam 4: Capacitance73 Questions
Exam 5: Electric Current and Direct-Current Circuits160 Questions
Exam 6: The Magnetic Field71 Questions
Exam 7: Sources of the Magnetic Field115 Questions
Exam 8: Magnetic Induction84 Questions
Exam 9: Alternating-Current Circuits119 Questions
Exam 10: Maxwells Equations and Electromagnetic Waves61 Questions
Exam 11: Properties of Light116 Questions
Exam 12: Optical Images143 Questions
Exam 13: Interference and Diffraction116 Questions
Exam 14: Wave Particle Duality and Quantum Physics153 Questions
Exam 15: Applications of the Schrodinger Equation54 Questions
Exam 16: Atoms128 Questions
Exam 17: Molecules44 Questions
Exam 18: Solids and the Theory of Conduction83 Questions
Exam 19: Relativity83 Questions
Exam 20: Nuclear Physics135 Questions
Exam 21: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
A gamma-ray photon of energy 800 keV scatters off an electron at an angle perpendicular to its original direction. It then scatters off a second electron such that this secondary scattered photon continues in the direction of the original photon. Calculate the energy difference between the initial and final photon.
(Multiple Choice)
4.9/5  (43)
(43)
Use the following figure for the next two questions. 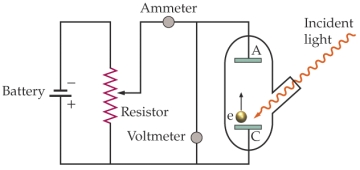 Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.5 * 10-19 J. The battery is set at 1.5 V. What is the longest wavelength of light needed to produce an electric current from the cathode (C) to the anode (A)?
Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.5 * 10-19 J. The battery is set at 1.5 V. What is the longest wavelength of light needed to produce an electric current from the cathode (C) to the anode (A)?
(Multiple Choice)
4.8/5  (37)
(37)
The pupil area of the human eye is ~ 1*10-5 m2. The minimum light intensity that the eye is sensitive to is ~ 1 *10-10 Wm-2. How many photons per second of wavelength 550 nm does this correspond to?
(Multiple Choice)
4.9/5  (37)
(37)
Light falling on the surface of a metal such as cesium can liberate electrons from the metal. The kinetic energy of electrons emitted from a metal can be increased by
(Multiple Choice)
4.8/5  (31)
(31)
An electron is in a one-dimensional box of length 0.5 nm. The ground-state energy of this electron is
(Multiple Choice)
4.8/5  (32)
(32)
An electron in the hydrogen atom (ground-state energy = -13.6 eV) makes a transition from the n = 2 to the n = 4 energy level. Calculate the magnitude of the energy of the photon involved in this process and state whether the photon was absorbed or emitted.
(Multiple Choice)
4.7/5  (37)
(37)
An electron is in a one-dimensional box of length 0.5 nm. The energy of this electron in its first excited state is
(Multiple Choice)
4.8/5  (37)
(37)
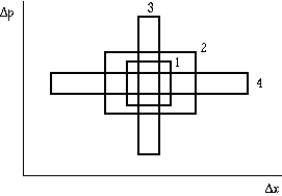 The graph shows the uncertainty in measuring the position x and momentum p of a particle in four experiments. Region 1 has area h, region 2 has area 4h, region 3 has area 2h, and region 4 has area 3h. The region that correctly represents the uncertainty principle is
The graph shows the uncertainty in measuring the position x and momentum p of a particle in four experiments. Region 1 has area h, region 2 has area 4h, region 3 has area 2h, and region 4 has area 3h. The region that correctly represents the uncertainty principle is
(Multiple Choice)
4.8/5  (36)
(36)
If the work function of thoriated tungsten is 4 *10-19 J, the longest wavelength of light that will cause photoelectrons to be emitted is approximately
(Multiple Choice)
4.8/5  (37)
(37)
The dissociation energy is the energy required to separate the two atoms in a diatomic molecule in their ground-state. If the dissociation energy of molecular oxygen is 7.2 eV, then calculate the maximum wavelength of light from the Sun that can break apart atmospheric O2.
(Multiple Choice)
4.9/5  (27)
(27)
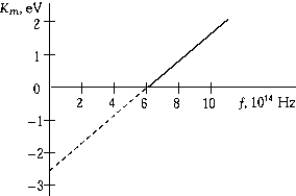 The graphs shows the maximum kinetic energy of electrons emitted from a metal as a function of the frequency of the incident light. The work function of the metal is
The graphs shows the maximum kinetic energy of electrons emitted from a metal as a function of the frequency of the incident light. The work function of the metal is
(Multiple Choice)
4.7/5  (30)
(30)
A particle is in the ground state of an infinite square-well potential. The probability of finding the particle in the region 0 < x < 3L/4 is
(Multiple Choice)
4.9/5  (40)
(40)
The wave function for a particle between x = -4 cm and 4 cm is given by 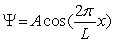 where L = 8 cm. Outside this range, the wave function is equal to zero. The probability of finding the particle between x = -2 cm and 2 cm is
where L = 8 cm. Outside this range, the wave function is equal to zero. The probability of finding the particle between x = -2 cm and 2 cm is
(Multiple Choice)
4.9/5  (40)
(40)
An electron in a hydrogen atom jumps from n = 5 to n = 2. The color of the photon given off is
(Multiple Choice)
4.7/5  (38)
(38)
Use the following equation statement to answer the next four questions.
The normalized wave functions for the infinite square-well potential are
n = (2/L)1/2 sin(n x/L)
You may find it useful to use 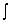 sin2 d = 2/4 - ( sin 2 /4 - (cos 2 /8 + C
-The expectation value <x> for a particle in the ground state of an infinite square-well potential is
sin2 d = 2/4 - ( sin 2 /4 - (cos 2 /8 + C
-The expectation value <x> for a particle in the ground state of an infinite square-well potential is
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following does not require the quantum theory of light for its explanation?
(Multiple Choice)
4.8/5  (35)
(35)
A proton is in a one-dimensional box of length ~ 2 * 10-15 m (i.e., ~ size of a nucleus). What is the approximate ground-state energy in eV?
(Multiple Choice)
4.9/5  (29)
(29)
Showing 101 - 120 of 153
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)