Exam 9: Covalent Bonding: Orbitals
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
Consider the skeletal structure shown below:
N-C-C-N
Draw the Lewis structure and answer the following:
-How many of the atoms are sp hybridized?
(Multiple Choice)
4.9/5  (35)
(35)
Whenever a set of equivalent tetrahedral atomic orbitals is required,an atom will adopt a set of sp3 orbitals.
(True/False)
4.8/5  (38)
(38)
A(n)__________ molecular orbital is lower in energy than the atomic orbital of which it is composed.
(Short Answer)
4.8/5  (38)
(38)
Which of the following are paramagnetic?
O2 O2- O22- B2 C2 N2 F2 CN- P2
(Essay)
4.9/5  (36)
(36)
In the molecule C2H4 the valence orbitals of the carbon atoms are assumed to be
(Multiple Choice)
4.7/5  (31)
(31)
Which of the following species has the largest dissociation energy?
(Multiple Choice)
4.8/5  (28)
(28)
Consider the molecule and the following hybridization choices: 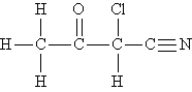 -What is the hybridization of the carbon atom that is double-bonded to oxygen?
-What is the hybridization of the carbon atom that is double-bonded to oxygen?
(Multiple Choice)
4.7/5  (30)
(30)
Tetracyanoethylene has the skeleton shown below: 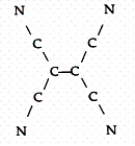 From its Lewis structure determine the following:
-How many nonbonded electron pairs are in the molecule?
From its Lewis structure determine the following:
-How many nonbonded electron pairs are in the molecule?
(Multiple Choice)
4.9/5  (36)
(36)
The H2- ion is more stable than H2 since it has an additional electron to produce a net lowering of energy.
(True/False)
4.8/5  (43)
(43)
The concept of __________ is required for certain molecules because the localized electron model assumes electrons are located between a given pair of atoms in a molecule.
(Short Answer)
4.8/5  (41)
(41)
Showing 21 - 40 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)