Exam 9: Covalent Bonding: Orbitals
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
If a molecule demonstrates paramagnetism,then :
I.The substance can have both paired and unpaired electrons.
II.The bond order is not a whole number.
III.It can be determined by drawing a Lewis structure.
IV.It must be an ion.
(Multiple Choice)
4.8/5  (30)
(30)
Which of the nitrogen-containing molecules below is paramagnetic in its lowest energy state?
(Multiple Choice)
4.8/5  (29)
(29)
Tetracyanoethylene has the skeleton shown below: 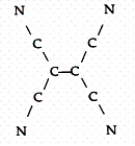 From its Lewis structure determine the following:
-How many of the atoms are sp hybridized?
From its Lewis structure determine the following:
-How many of the atoms are sp hybridized?
(Multiple Choice)
4.9/5  (43)
(43)
Explain the concept of delocalization of electrons in SO3.Indicate how this idea relates to resonance.
(Essay)
4.8/5  (41)
(41)
Which of the following statements about the species CN- is false?
(Multiple Choice)
4.9/5  (39)
(39)
For how many of the following does the bond order decrease if you add one electron to the neutral molecule? B2,C2,P2,F2
(Multiple Choice)
4.9/5  (39)
(39)
Draw a molecular orbital diagram for O2 and N2.Using molecular orbital theory,explain why the removal of one electron in O2 strengthens bonding,while the removal of one electron in N2 weakens bonding.
(Essay)
4.8/5  (33)
(33)
As the bond order of a bond increases,the bond energy ______ and the bond length ______.
(Multiple Choice)
4.8/5  (37)
(37)
When an electron pair is shared in the area centered on a line joining the atoms,a (sigma)bond is formed.
(True/False)
4.9/5  (40)
(40)
Consider the skeletal structure shown below:
N-C-C-N
Draw the Lewis structure and answer the following:
-How many pi bonds does the molecule contain?
(Multiple Choice)
4.8/5  (46)
(46)
The hybridization of a molecule is measured to determine the shape of the molecule.
(True/False)
4.8/5  (38)
(38)
A species has the following MO configuration: ( 1s)2( 1s*)2( 2s)2( 2s*)2( 2p)2( 2p)2
This substance is
(Multiple Choice)
4.9/5  (36)
(36)
Consider the molecule and the following hybridization choices: 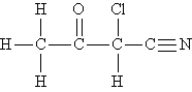 -What is the hybridization of the nitrogen atom?
-What is the hybridization of the nitrogen atom?
(Multiple Choice)
4.8/5  (34)
(34)
For which of the following diatomic molecules would the bond order become greater if an electron is removed (i.e. ,if the molecule is converted to the positive ion in its ground state)?
(Multiple Choice)
4.8/5  (32)
(32)
__________ is the difference between the number of bonding electrons and the number of antibonding electrons divided by two.
(Short Answer)
4.9/5  (41)
(41)
Showing 41 - 60 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)