Exam 9: Covalent Bonding: Orbitals
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
The following statements concern molecules that require resonance.Which is true?
(Multiple Choice)
4.9/5  (38)
(38)
Consider the following Lewis structure: 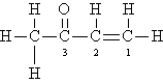 Which statement about the molecule is false?
Which statement about the molecule is false?
(Multiple Choice)
5.0/5  (32)
(32)
When comparing Be2 and H2:
I.Be2 is more stable because it contains both bonding and antibonding valence electrons.
II.H2 has a higher bond order than Be2.
III.H2 is more stable because it only contains 1s electrons.
IV.H2 is more stable because it is diamagnetic,whereas Be2 is paramagnetic.
(Multiple Choice)
4.8/5  (37)
(37)
Consider the molecule and the following hybridization choices: 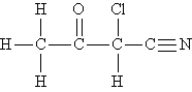 -What is the hybridization of the carbon atom that is bonded to chlorine?
-What is the hybridization of the carbon atom that is bonded to chlorine?
(Multiple Choice)
4.8/5  (33)
(33)
Consider the following Lewis structure: 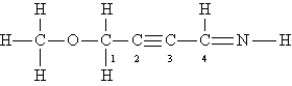 What is the hybridization of the atoms O,C-1,C-2,and C-4?
What is the hybridization of the atoms O,C-1,C-2,and C-4?
(Multiple Choice)
4.9/5  (34)
(34)
Consider the benzene molecule.Which of the following statements about the molecule is false?
(Multiple Choice)
4.8/5  (42)
(42)
Consider the molecule and the following hybridization choices:  -What is the hybridization of the oxygen atom?
-What is the hybridization of the oxygen atom?
(Multiple Choice)
4.7/5  (43)
(43)
Complete the Lewis structure for the following molecule: 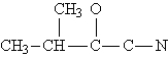 This molecule has __________ sigma and __________ pi bonds.
This molecule has __________ sigma and __________ pi bonds.
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following statements about the CO32- ion is false?
(Multiple Choice)
4.9/5  (48)
(48)
Which of the following molecules or ions is not paramagnetic in its ground state?
(Multiple Choice)
4.8/5  (39)
(39)
The electron configuration of a particular diatomic species is ( 2s)2( *2s)2( 2p)2( 2p)4( *2p)2.What is the bond order for this species?
(Multiple Choice)
4.8/5  (43)
(43)
If four orbitals on one atom overlap four orbitals on a second atom,how many molecular orbitals will form?
(Multiple Choice)
5.0/5  (37)
(37)
Use the molecules below to answer the next three questions. 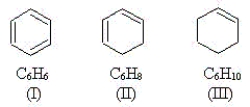 -Which molecule(s)have at least one carbon atom that is sp hybridized?
-Which molecule(s)have at least one carbon atom that is sp hybridized?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 81 - 100 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)