Exam 7: Atomic Structure and Periodicity
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
Choose the atom or ion using a periodic table.
-Larger atomic radius,P or Sb
(Short Answer)
4.8/5  (31)
(31)
Consider a planet where the temperature is so high that the ground state of an electron in the hydrogen atom is n = 4.What is the ratio of ionization energy for hydrogen on this planet compared to that on Earth?
(Multiple Choice)
4.8/5  (30)
(30)
Order the elements S,Cl,and F in terms of increasing atomic radii.
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following could not be a valid ml quantum number for a 4d orbital?
(Multiple Choice)
4.9/5  (37)
(37)
Consider the graph below to answer the next two questions: 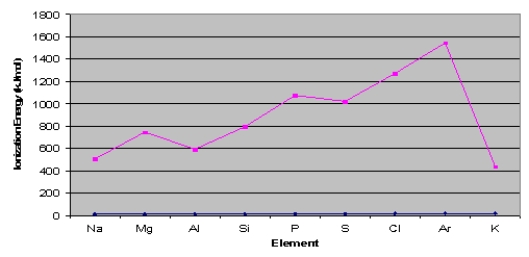 -Explain the ionization energy difference between sodium and potassium.
-Explain the ionization energy difference between sodium and potassium.
(Essay)
4.7/5  (38)
(38)
Give the quantum numbers for the last electron in:
a)gold
b)magnesium
c)iodine
d)cadmium
(Essay)
4.7/5  (35)
(35)
Which of the following is not determined by the principal quantum number,n,of the electron in a hydrogen atom?
(Multiple Choice)
4.9/5  (34)
(34)
What is the wavelength of a photon of red light (in nm)whose frequency is 4.55 1014 Hz?
(Multiple Choice)
4.8/5  (39)
(39)
The number of orbitals having a given value of l is equal to
(Multiple Choice)
4.8/5  (26)
(26)
An element has the electron configuration [Kr] 5s24d105p2.The element is a(n)
(Multiple Choice)
4.7/5  (39)
(39)
Consider the graph below to answer the next two questions:  -Explain why argon has the highest ionization energy.
-Explain why argon has the highest ionization energy.
(Essay)
4.7/5  (40)
(40)
The small,but important,energy differences between 3s,3p,and 3d orbitals are due mainly to
(Multiple Choice)
4.7/5  (37)
(37)
For the set of elements Li,O,Ne,and Na,which element has the largest atomic radius? Explain any deviation from the expected pattern.
(Essay)
4.9/5  (36)
(36)
Choose the atom or ion using a periodic table.
-Larger first ionization energy,Na or Rb
(Short Answer)
4.8/5  (41)
(41)
Showing 81 - 100 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)