Exam 7: Atomic Structure and Periodicity
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
Which of the following processes represents the ionization energy of bromine?
(Multiple Choice)
4.9/5  (43)
(43)
From the following list of observations,choose the one that most clearly supports the following conclusion:
a)emission spectrum of hydrogen
b)the photoelectric effect
c)scattering of alpha particles by metal foil
d)diffraction
e)cathode "rays"
-Electromagnetic radiation has wave characteristics.
(Multiple Choice)
4.7/5  (40)
(40)
The statement that "the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate orbitals" is known as
(Multiple Choice)
4.8/5  (33)
(33)
How many electrons in an atom can have the following quantum numbers?
a)n = 3
b)n = 2,l = 0
c)n = 2,l = 2,ml = 0
d)n = 2,l = 0,ml = 0,ms = 1/2
(Essay)
4.8/5  (38)
(38)
A line in the spectrum of atomic mercury has a wavelength of 254 nm.When mercury emits a photon of light at this wavelength,the frequency of this light is
(Multiple Choice)
4.8/5  (36)
(36)
Fe has __________ that is (are)unpaired in its d orbitals.
(Multiple Choice)
4.7/5  (35)
(35)
Consider the following representation of a 2p-orbital: 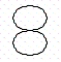 Which of the following statements best describes the movement of electrons in a p-orbital?
Which of the following statements best describes the movement of electrons in a p-orbital?
(Multiple Choice)
4.9/5  (47)
(47)
List the following atoms in order of increasing ionization energy: Li,Na,C,O,F.
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following is a reasonable criticism of the Bohr model of the atom?
(Multiple Choice)
4.8/5  (38)
(38)
The __________ quantum number is related to the size and energy of the orbital.
(Short Answer)
4.8/5  (46)
(46)
Of the following elements,which needs three electrons to complete its valence shell?
(Multiple Choice)
4.8/5  (37)
(37)
Choose the atom or ion using a periodic table.
-Larger atomic or ionic radius,F or F-
(Short Answer)
4.8/5  (43)
(43)
When examining the electromagnetic spectrum,why is it more harmful to be exposed to x-rays than radio waves over a period of time? In your explanation,include the concepts of frequency,waves,and energy.Also,draw transverse waves to assist in your explanation.
(Essay)
4.8/5  (45)
(45)
Which of the following statements about quantum theory is incorrect?
(Multiple Choice)
4.7/5  (30)
(30)
Given the following electronic configuration of neutral atoms,identify the element and state the number of unpaired electrons in its ground state:
-[Ne]3s23p5
(Essay)
4.7/5  (45)
(45)
When electron configurations differ from expected,it is because orbitals want to be half-filled.
(True/False)
4.9/5  (35)
(35)
Showing 121 - 140 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)