Exam 11: Properties of Solutions
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
How many molecules of sucrose (table sugar),C12H22O11,dissolved in 450.0 g of water are needed to make a 1.81 m solution?
(Multiple Choice)
4.9/5  (46)
(46)
The osmotic pressure of a 0.0100 M solution of NaCl in water at 25°C is found to be different from 372 torr because:
(Multiple Choice)
4.8/5  (39)
(39)
Find the mass percent of CuSO4 in a solution whose density is 1.30 g/mL and whose molarity is 1.36 M.
(Multiple Choice)
4.7/5  (43)
(43)
For each of the following solutions,describe the deviation with respect to Raoult's Law.
-acetone (C3H6O)and water
(Multiple Choice)
4.8/5  (31)
(31)
The vapor pressure of water at 25.0°C is 23.8 torr.Determine the mass of glucose (molar mass = 180 g/mol)needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
(Multiple Choice)
4.9/5  (35)
(35)
Use the following drawing of a gaseous solute in equilibrium with a solution to help answer the question below. 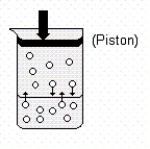 Which of the following statements are true when the piston is pushed in (downward)?
Which of the following statements are true when the piston is pushed in (downward)?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following chemical or physical changes is an endothermic process?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 101 - 108 of 108
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)