Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions
Exam 1: Structure and Bonding:acids and Bases40 Questions
Exam 2: Alkanes: The Nature of Organic Compounds47 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions37 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds51 Questions
Exam 6: Sterechemistry at Tetrahedral Centers35 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations49 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs39 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions66 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions37 Questions
Exam 12: Amines31 Questions
Exam 13: Structure Determination64 Questions
Exam 14: Biomolecules: Carbohydrates41 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins43 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids37 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways35 Questions
Select questions type
Instructions: Predict the products from the information given for the following question(s).
Predict: 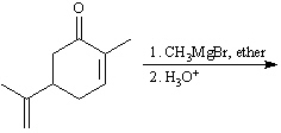
(Essay)
4.8/5  (32)
(32)
Instructions: a,b-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the b carbon, as shown below. Use this information to answer the following question(s). 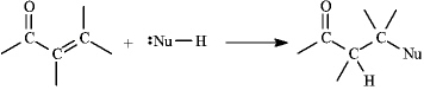 Refer to instructions. This reaction is called a(n) _____ reaction.
Refer to instructions. This reaction is called a(n) _____ reaction.
(Multiple Choice)
4.7/5  (30)
(30)
Propose a synthesis of Dimestrol starting from p-methoxypropiophenone as the only source of carbon.
(Essay)
4.7/5  (43)
(43)
Instructions: Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated. 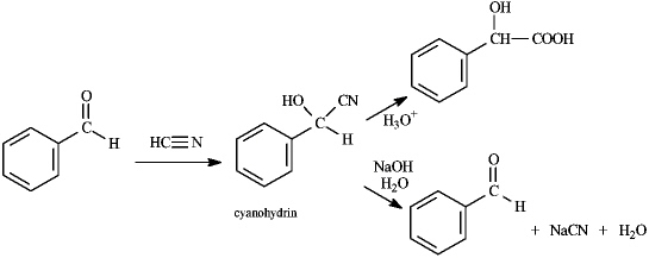 Refer to instructions. Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
Refer to instructions. Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
(Essay)
4.8/5  (27)
(27)
Instructions: Predict the products from the information given for the following question(s).
Predict: 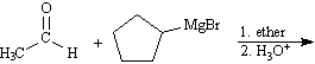
(Essay)
4.8/5  (41)
(41)
Instructions: a,b-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the b carbon, as shown below. Use this information to answer the following question(s). 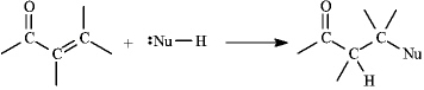 Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
(Essay)
4.8/5  (33)
(33)
What is the correct structure for 2-hydroxyacetophenone? a. 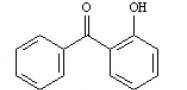 b.
b. 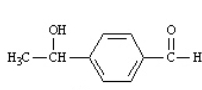 c.
c. 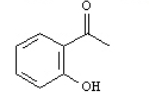 d.
d. 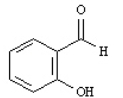
(Short Answer)
4.8/5  (36)
(36)
Instructions: Predict the products from the information given for the following question(s).
Predict: 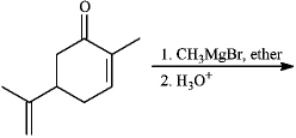
(Essay)
4.7/5  (35)
(35)
Instructions: Predict the products from the information given for the following question(s).
Predict: 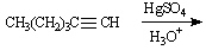
(Essay)
4.9/5  (33)
(33)
What hemiacetal would form from the internal nucleophillic addition reaction of the following compound? 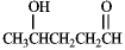
(Essay)
4.9/5  (38)
(38)
Showing 21 - 32 of 32
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)