Exam 2: Atoms, Ions, and Compounds
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
For each of the elements below, there are only two naturally occurring isotopes. Using information in your periodic table, identify the pair in which the heavier isotope is the more abundant one.
(Multiple Choice)
4.8/5  (28)
(28)
Sodium nitrite, which is used in meat processing, has been implicated as a possible health hazard because it can react with amines present in meat to form trace quantities of carcinogenic nitrosamines. What is the formula of sodium nitrite?
(Multiple Choice)
4.9/5  (47)
(47)
Name the following oxides of nitrogen in this sequence: NO, N2O, NO2, N2O4.
(Multiple Choice)
4.8/5  (35)
(35)
Iodine-137 decays to xenon by beta emission, which then decays to cesium-137. Write the nuclear reaction equations for these two decay processes.
(Essay)
4.7/5  (40)
(40)
Which particle-level diagram is the best representation of a 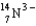 ion?
ion? 
(Multiple Choice)
4.7/5  (44)
(44)
The evaporation of seawater gives a mixture of ionic compounds containing sodium combined with chloride, sulfate, carbonate, and hydrogen carbonate. Write the chemical formulas of these compounds.
(Essay)
4.8/5  (33)
(33)
If an atom had a radius of 100 m, it would be approximately the size of a football stadium. On this scale, what would be the radius of the atomic nucleus since the radius of the nucleus is approximately 10,000 times smaller than the radius of an atom?
(Multiple Choice)
4.9/5  (40)
(40)
What is the correct formula for the compound formed between sodium and iodine based on their positions in the periodic table?
(Multiple Choice)
4.8/5  (39)
(39)
The average atomic mass of calcium is 40.078 amu. Which is the most abundant isotope of calcium?
(Multiple Choice)
4.9/5  (40)
(40)
Buffer solutions that maintain certain levels of pH or acidity are widely used in biochemical experiments. One common buffer system uses sodium dihydrogenphosphate and sodium monohydrogenphosphate. What are the formulas of these two compounds?
(Multiple Choice)
4.9/5  (40)
(40)
Zinc oxide is found in ointments for the skin. What formula best describes this compound, which has Zn as a doubly charged cation?
(Multiple Choice)
4.8/5  (32)
(32)
Nitrogen and oxygen combine to form several different nitrogen oxides. Chemical analysis found that the N:O mass ratio in NO is 0.875. Two other nitrogen oxides were produced by reacting 8.4 g of nitrogen completely with 4.8 g of oxygen in one case and in another case by reacting 4.2 g of nitrogen with 9.6 g of oxygen. What are the empirical formulas of these two nitrogen oxides?
(Short Answer)
4.8/5  (35)
(35)
Showing 21 - 40 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)