Exam 2: Atoms, Ions, and Compounds
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which of the following is most likely to exhibit covalent bonding?
(Multiple Choice)
4.8/5  (33)
(33)
The average atomic mass of lithium is 6.941 amu. Lithium has two naturally occurring isotopes, 6Li (7.52%) and 7Li (92.48%). The mass of 6Li is 6.0151 amu. What is the mass of 7Li?
(Multiple Choice)
4.9/5  (31)
(31)
Enriched weapons-grade uranium consists of 80% uranium-235 (235.044 amu) and 20% uranium-238 (238.051 amu). What is the average atomic mass of weapons grade uranium assuming the percentages are exact?
(Multiple Choice)
4.8/5  (47)
(47)
Which one of the following is a molecular compound? Molecular compounds also are known as covalent compounds.
(Multiple Choice)
4.8/5  (32)
(32)
A 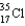 atom has __________ protons, __________ neutrons, and __________ electrons.
atom has __________ protons, __________ neutrons, and __________ electrons.
(Multiple Choice)
4.9/5  (48)
(48)
Which would produce the larger mass of carbon dioxide, CO2, when combined with oxygen: 1 g of pure carbon or 1 g of pure octane (C8H14)? Assume that all the carbon is converted to carbon dioxide in both cases.
(Multiple Choice)
4.8/5  (30)
(30)
Which particle diagram is the best representation for a 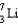 atom?
atom? 
(Multiple Choice)
4.7/5  (44)
(44)
For each of the elements below, there are only two naturally occurring isotopes. Using information in your periodic table, identify the pair in which the lighter isotope is the more abundant one.
(Multiple Choice)
4.9/5  (33)
(33)
The emission of a particle is associated with the __________
(Multiple Choice)
4.9/5  (33)
(33)
Iron can form two sulfides: FeS and Fe2S3. Use Dalton's law of multiple proportions to predict the ratio of the two masses of sulfur that combine with 100 g of iron in each case to form these compounds.
(Multiple Choice)
4.9/5  (45)
(45)
Aqua regia is a mixture of hydrochloric acid and nitric acid that is capable of dissolving gold. What are the formulas of these acids?
(Multiple Choice)
4.8/5  (38)
(38)
Write the complete atomic symbol with both a superscript and a subscript for a sodium ion that contains 11 protons, 10 electrons, and 12 neutrons.
(Short Answer)
4.9/5  (36)
(36)
Showing 61 - 80 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)