Exam 17: Additional Aspects of Acid-Base Equilibria
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
Phenol red indicator changes from yellow to red in the pH range from 6.6 to 8.0. State what color the indicator will assume in the following solution: 0.10 M NH4NO3
(Multiple Choice)
4.8/5  (38)
(38)
Twenty-five milliliters of 0.10 M HCl is titrated with 0.10 M NaOH. What is the pH at equivalence?
(Multiple Choice)
4.8/5  (35)
(35)
What is the pH of a 1.0 M solution of Na3AsO4?( Ka1 = 6 × 10-3, Ka2 = 1 × 10-7, Ka3 = 3 × 10-12 )
(Multiple Choice)
4.9/5  (42)
(42)
What is the pH at the equivalence point for the titration of 0.20 M nitrous acid by 0.20 M sodium hydroxide? [Ka for nitrous acid is 4.5 × 10-4]
(Multiple Choice)
4.8/5  (40)
(40)
Twenty-five milliliters of 0.10 M HCl is titrated with 0.10 M NaOH. What is the pH when 30 ml of NaOH have been added?
(Multiple Choice)
4.8/5  (35)
(35)
For an accurate titration, the end point needs to match the equivalence point.
(True/False)
4.9/5  (41)
(41)
25 ml of 0.10 M acetic acid is titrated with 0.10 M NaOH. What is the pH at the equivalence point? Ka for acetic acid = 1.8 × 10-5.
(Multiple Choice)
4.9/5  (30)
(30)
1.80 grams of an impure mixture containing sodium carbonate required 84.0 ml of 0.125 M H2SO4 for complete neutralization. What percent of the mixture is sodium carbonate?
(Multiple Choice)
4.8/5  (39)
(39)
What will the pH at the neutralization point of 0.00812 M Ba(OH)2 be when titrated with HCl?
(Multiple Choice)
4.7/5  (44)
(44)
Determine the pH of the following solution. Initial concentrations are given. [HF] = 1.296 M, [HCl] = 1.045 M, Ka for HF is 6.6 × 10-4
(Multiple Choice)
4.9/5  (34)
(34)
What is the pH of a 1.0 M solution of trisodium phosphate? Ka1 = 7.1 × 10-3, Ka2 = 6.3 × 10-8, Ka3 = 4.2 × 10-13
(Multiple Choice)
4.7/5  (39)
(39)
Determine the pH of the following solution. Initial concentrations are given. [NaCHO2] = 0.815 M, [HBr] = 0.105 M, Ka (HCHO2) = 1.8 × 10-4
(Multiple Choice)
4.7/5  (37)
(37)
Determine the pH of the following solution. Initial concentrations are given. [HC2H3O2] = 0.250 M, [NaC2H3O2] = 0.120 M [Ka = 1.8 × 10-5]
(Multiple Choice)
4.7/5  (28)
(28)
What volume in ml of 0.05 M NaOH would have to be added to 50.0 ml of 0.10 M H2SO4 in order to affect complete neutralization of the acid?
(Multiple Choice)
4.8/5  (45)
(45)
For the following titration, determine whether the solution at the equivalence point is acidic, basic, or neutral and why: NaHCO3(aq) titrated with NaOH(aq)
(Multiple Choice)
4.8/5  (34)
(34)
Phenolphthalein may be used as an indicator for the titration of:
(Multiple Choice)
4.7/5  (32)
(32)
A solution of sodium carbonate is easier to calculate the pH than sodium hydrogen carbonate because there is only one hydrolysis reaction instead of two.
(True/False)
4.8/5  (46)
(46)
The titration curve for 10.0 mL of 0.100 M H3PO4(aq) with 0.100 M NaOH(aq) is given below. 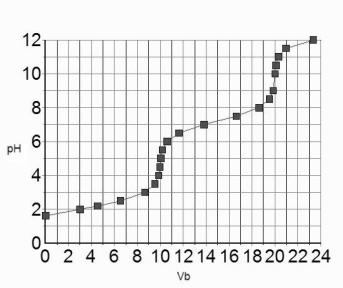 Estimate the pKa2 of H3PO4.
Estimate the pKa2 of H3PO4.
(Multiple Choice)
4.8/5  (40)
(40)
Showing 41 - 60 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)