Exam 11: Chemical Bonding II: Additional Aspects
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
Find the correct statements about the bonding in methane, CH4.
I. The carbon s and p orbitals combine to form four equivalent sp3 orbitals
II. All C-H bonds have the same strength
III. Molecular geometry is different from electron group geometry
IV. Four C sp3 orbitals combine with the s orbitals of the hydrogens to form bonds
(Multiple Choice)
4.8/5  (40)
(40)
Which is the correct molecular orbital diagram for carbon monoxide?
(Multiple Choice)
4.9/5  (35)
(35)
The structure of aspirin is given below. 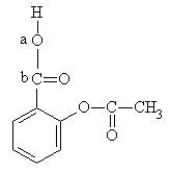 Which set of hybrid orbitals best describes the O-C bond, labeled "a", "b", in aspirin?
Which set of hybrid orbitals best describes the O-C bond, labeled "a", "b", in aspirin?
(Multiple Choice)
4.8/5  (32)
(32)
The inclusion of a small amount of aluminum in a crystal of pure germanium yields what kind of semiconductor?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following carbon molecules has sp hybridization?
(Multiple Choice)
4.8/5  (42)
(42)
The double covalent bond between two carbon atoms in ethylene (C2H4):
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following could act as a p-type semiconductor?
(Multiple Choice)
4.8/5  (32)
(32)
How many π-electrons are there in S2O where S is the central atom?
(Multiple Choice)
4.9/5  (37)
(37)
If the HCOO- ion is described using delocalized electrons, why can the oxygen atoms not have sp3 hybrids?
(Multiple Choice)
4.9/5  (39)
(39)
For XeF4, what are the dipole moment orientation, hybridization on the central atom, and the number of lone pairs on the central atom?
(Multiple Choice)
4.7/5  (34)
(34)
The HCOO- ion can be described by using delocalized electrons. What is the hybridization of the C atom?
(Multiple Choice)
4.8/5  (36)
(36)
The description of covalent bond formation as a region of high electronic charge density resulting from overlap of atomic orbitals between the two bonded atoms is referred to as:
(Multiple Choice)
4.9/5  (40)
(40)
Which combination of hybrid orbital descriptions and electronic geometry descriptions is INCORRECT?
(Multiple Choice)
4.7/5  (33)
(33)
How many σ bonds and how many π bonds are present in the carbonic acid molecule, H2CO3?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 21 - 40 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)