Exam 11: Chemical Bonding II: Additional Aspects
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
Which is the correct molecular orbital diagram for O2- ion?
(Multiple Choice)
4.7/5  (47)
(47)
Which of the following is INCORRECT about silicon doped with phosphorus?
(Multiple Choice)
4.7/5  (30)
(30)
According to Molecular Orbital (MO) theory, which statement below is true?
(Multiple Choice)
4.9/5  (43)
(43)
The inclusion of a small amount of phosphorus in a crystal of pure germanium yields what kind of semiconductor?
(Multiple Choice)
4.8/5  (39)
(39)
If the wave functions describing the 2s and two of the 2p orbitals are combined, the identical orbitals derived form bond angles of ________.
(Multiple Choice)
4.7/5  (38)
(38)
According to the valence bond theory, in the ion NH4+, nitrogen is using which orbitals for bonding?
(Multiple Choice)
4.9/5  (39)
(39)
One resonance structure of N2O is 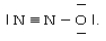 The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
(Multiple Choice)
4.8/5  (31)
(31)
How many σ- and π-bonds, respectively, are there in phenylisothiocyanate, C6H5NCS? Ignore the C-H bonds in your counting.
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following statements concerning the relative energy levels of molecular orbitals for the O2 molecule is INCORRECT?
(Multiple Choice)
4.8/5  (34)
(34)
According to the principles of VSEPR applied on ICl5, which of the following is INCORRECT?
(Multiple Choice)
4.8/5  (35)
(35)
According to the molecular orbital (MO) theory, when two oxygen atoms bond together, their 2p orbitals combine to form:
(Multiple Choice)
4.8/5  (39)
(39)
Showing 61 - 80 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)