Exam 9: Chemical Reactions
Exam 1: Basic Concepts About Matter70 Questions
Exam 2: Measurements in Chemistry44 Questions
Exam 3: Atomic Structure and the Periodic Table70 Questions
Exam 4: Chemical Bonding: the Ionic Bond Model70 Questions
Exam 5: Chemical Bonding: the Covalent Bond Model70 Questions
Exam 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations70 Questions
Exam 7: Gases, Liquids, and Solids65 Questions
Exam 8: Solutions69 Questions
Exam 9: Chemical Reactions66 Questions
Exam 10: Acids, Bases, and Salts70 Questions
Exam 11: Nuclear Chemistry70 Questions
Exam 12: Saturated Hydrocarbons70 Questions
Exam 13: Unsaturated Hydrocarbons70 Questions
Exam 14: Alcohols, Phenols, and Ethers70 Questions
Exam 15: Aldehydes and Ketones66 Questions
Exam 16: Carboxylic Acids, Esters, and Other Acid Derivatives64 Questions
Exam 17: Amines and Amides54 Questions
Exam 18: Carbohydrates70 Questions
Exam 19: Lipids70 Questions
Exam 20: Proteins65 Questions
Exam 21: Enzymes and Vitamins70 Questions
Exam 22: Nucleic Acids64 Questions
Exam 23: Biochemical Energy Production70 Questions
Exam 24: Carbohydrate Metabolism70 Questions
Exam 25: Lipid Metabolism65 Questions
Exam 26: Protein Metabolism70 Questions
Select questions type
For the indicated element, select the correct oxidation number from the response list: oxidation number of Mn in Mn3+.
(Multiple Choice)
4.9/5  (30)
(30)
Most reactions are carried out in liquid solution or in the gaseous phase because in such situations
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following changes is most likely to decrease reaction rate for the chemical reaction 2CO + O2 2CO2?
(Multiple Choice)
4.9/5  (27)
(27)
For the indicated element, select the correct oxidation number from the response list: oxidation number of S in H2SO4+.
(Multiple Choice)
4.9/5  (35)
(35)
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
-Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Electron loss is always associated with an increase in oxidation number.
(2) An exothermic reaction occurs when the energy required to break bonds in reactants is less than the energy released by bond formation in the products.
(3) The concentrations of pure liquids and pure solids are never included in an equilibrium constant expression because such concentrations never change.
(Multiple Choice)
4.9/5  (38)
(38)
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
-Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
(1) All chemical systems will reach equilibrium if enough time elapses.
(2) A substance that functions as a reducing agent is, itself, oxidized.
(3) In a slow reaction, the activation energy is far greater than the average energy content of the reacting particles.
(Multiple Choice)
4.9/5  (42)
(42)
Assign the following reaction to one of the reaction classifications given in the response list: 2NO2 + H2O2 2HNO3
(Multiple Choice)
4.8/5  (40)
(40)
The proper assignment of oxidation numbers to the elements in the compound LiAsO3 would be
(Multiple Choice)
4.8/5  (33)
(33)
CO2 and H2 are allowed to react until equilibrium is established as follows:  Which of the following changes will cause the equilibrium position to shift to the right?
Which of the following changes will cause the equilibrium position to shift to the right?
(Multiple Choice)
4.8/5  (42)
(42)
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
-Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The collision between two reactant molecules does not always result in the formation of reaction products.
(2) The substance oxidized in a reaction may be either a reactant or a product.
(3) At chemical equilibrium, the concentrations of reactants and products cease to change.
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following statements concerning oxidation and reduction is correct?
(Multiple Choice)
4.9/5  (36)
(36)
Consider the following redox equation: SO2 + NO2 SO3 + NO, choose an appropriate chemical formula from the list that identifies the oxidizing agent.
(Multiple Choice)
4.8/5  (39)
(39)
Assign the following reaction to one of the reaction classifications given in the response list: Mg + 2HCl MgCl2 + H2
(Multiple Choice)
4.7/5  (39)
(39)
In writing an equilibrium constant expression, which of the following is incorrect?
(Multiple Choice)
4.8/5  (35)
(35)
According to Le Chatelier's principle, which of the following changes will shift the position of the equilibrium to the left for the reaction 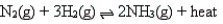
(Multiple Choice)
4.7/5  (26)
(26)
The minimum combined kinetic energy reactant particles must possess in order for their collision to result in a reaction is called the
(Multiple Choice)
4.9/5  (41)
(41)
Consider the following equilibrim:  Determine the effect on the position of equilibrium when a catalyst is added.
Determine the effect on the position of equilibrium when a catalyst is added.
(Multiple Choice)
4.9/5  (38)
(38)
Consider the following equilibrim:  Determine the effect on the position of equilibrium when some H2 is added.
Determine the effect on the position of equilibrium when some H2 is added.
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following statements concerning redox reactions is incorrect?
(Multiple Choice)
4.9/5  (27)
(27)
Showing 21 - 40 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)