Exam 9: Acids and Bases
Exam 1: Matter and Measurement89 Questions
Exam 2: Atoms and the Periodic Table90 Questions
Exam 3: Ionic Compounds90 Questions
Exam 4: Covalent Compounds90 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: Energy Changes, Reaction Rates, and Equilibrium88 Questions
Exam 7: Gases, Liquids, and Solids79 Questions
Exam 8: Solutions90 Questions
Exam 9: Acids and Bases90 Questions
Exam 10: Nuclear Chemistry85 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups103 Questions
Exam 12: Alkanes106 Questions
Exam 13: Unsaturated Hydrocarbons101 Questions
Exam 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur111 Questions
Exam 15: The Three-Dimensional Shape of Molecules100 Questions
Exam 16: Aldehydes and Ketones103 Questions
Exam 17: Carboxylic Acids, Esters, and Amides81 Questions
Exam 18: Amines and Neurotransmitters105 Questions
Exam 19: Lipids105 Questions
Exam 20: Carbohydrates92 Questions
Exam 21: Amino Acids, Proteins, and Enzymes88 Questions
Exam 22: Nucleic Acids and Protein Synthesis89 Questions
Exam 23: Digestion and the Conversion of Food Into Energy92 Questions
Exam 24: Carbohydrate, Lipid, and Protein Metabolism91 Questions
Select questions type
Normal gastric juice has a pH of about 2. Assuming that normal gastric juice is primarily aqueous HCl, what is the concentration of HCl in the stomach?
(Multiple Choice)
4.9/5  (28)
(28)
Which diagram properly depicts the dissolved species present in an aqueous solution of KOH? (water molecules are not shown)
(Multiple Choice)
4.8/5  (34)
(34)
HPO42- is amphoteric. The conjugate acid of HPO42- is _____ and its conjugate base is _____.
(Essay)
4.8/5  (35)
(35)
Which term correctly describes the medical condition in which the pH of blood is greater than 7.45, and therefore the blood is more basic than normal?
(Multiple Choice)
4.9/5  (37)
(37)
Which buffer solution has the lowest pH (HF has Ka = 7.2 × 10-4)?
(Multiple Choice)
4.8/5  (39)
(39)
The addition of an acid to water increases the [H3O+] of the solution and increases the solution pH.
(True/False)
4.8/5  (41)
(41)
In the reaction: SO32-(aq)+ H2O(l) 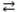 HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
(True/False)
4.8/5  (29)
(29)
The products are favored in the acid-base reaction: HI(aq)+ NH3(aq) 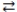 NH4+(aq)+ I-(aq).
NH4+(aq)+ I-(aq).
(True/False)
4.8/5  (41)
(41)
An aqueous solution whose pH is greater than 7 has a higher [H3O+] than pure water itself.
(True/False)
4.8/5  (33)
(33)
An aqueous solution containing an equal number of moles of NaF and HF is an example of a buffer solution.
(True/False)
4.8/5  (33)
(33)
Which solution containing an equal number of moles of each of the substances is a buffer?
(Multiple Choice)
4.8/5  (42)
(42)
The principal buffer in the blood is carbonic acid/bicarbonate (H2CO3/HCO3-), keeping the normal blood pH of a healthy individual in the range of 7.35 to 7.45.
(True/False)
4.8/5  (38)
(38)
An aqueous solution containing an equal number of moles of Na2HPO4 and H3PO4 is an example of a buffer solution.
(True/False)
4.8/5  (32)
(32)
If two solutions differ in their [H3O+] by a factor of 2.0, the difference in their pH will be 2.0.
(True/False)
4.8/5  (39)
(39)
Showing 41 - 60 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)